Ch4 boiling point celsius
Home » datasheet » Ch4 boiling point celsiusCh4 boiling point celsius
Ch4 Boiling Point Celsius. It is one state of water within the hydrosphere. Or pT Constant. We can select a range of elements too. 1H naturally plenty 999985 percent.
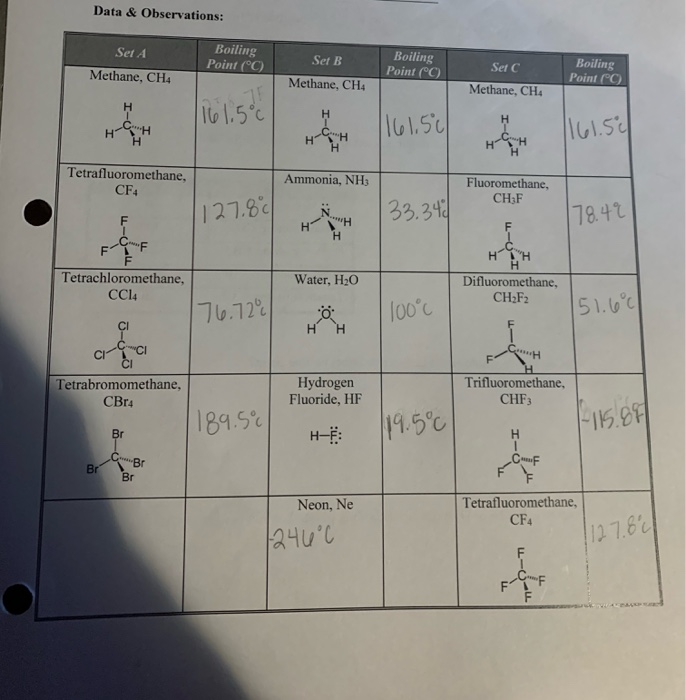 Solved Data Observations Boiling Point C Set A Boiling Chegg Com From chegg.com
Solved Data Observations Boiling Point C Set A Boiling Chegg Com From chegg.com
What is the strongest type of intermolecular force present in NH2CH3. At the boiling point of a liquid the vapor pressure equals the external usually atmospheric pressure. The letter T represents Kelvin or absolute temperatures and θ stands for a Celsius scale temperature. When water is the solvent the boiling point of water will increase 0512C for each 76 grams of propylene glycol antifreeze added to 1000 grams of water. Boiling point - 423K Vapour pressure 298K 19mmHg. It is one state of water within the hydrosphere.
The bubble point is the saturated liquid state in a mixture where the first bubble appears ie the onset of boiling.
It means that 1ml of 30 H2O2 solution will give 100V of oxygen at STP. Calculate the molar mass of the solute. None of the above. The pressure of a fixed mass of gas is directly proportional to its absolute temperature if the volume is kept constant. The dew point is the saturated vapor state in a mixture where the first droplet of liquid appears ie the onset of condensation. 1705 mmHg 844 mmHg _ c.
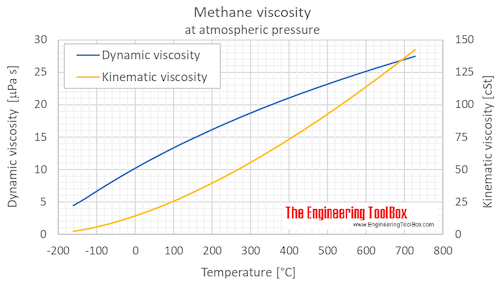 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Sicl4 structure. The freezing point of water will decrease 186C for. What structural features are present that could possibly make formation of the 14 product more favourable than the 12 product even though it goes through a less stable carbocation. Freezing point depression boiling point elevation vapor pressure lowering and osmotic pressure are all colligative properties. It is currently being applied in many industries from chemical and refining to.
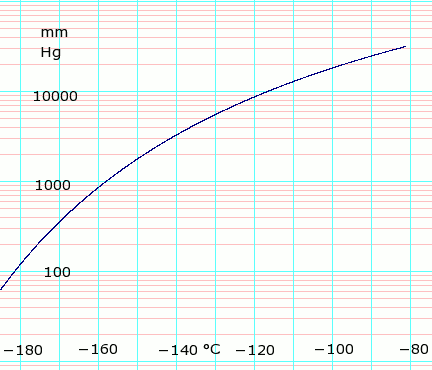 Source: en.wikipedia.org
Source: en.wikipedia.org
The freezing point of water will decrease 186C for. With Butadiene The 14 Product Is More Stable Because It Has A More Substituted Double Bond. The pressure of a fixed mass of gas is directly proportional to its absolute temperature if the volume is kept constant. This pressure decreases by 198 mmHg for every. At the boiling point of a liquid the vapor pressure equals the external usually atmospheric pressure.
 Source: clutchprep.com
Source: clutchprep.com
The image below shows two trends based on NASA 1880-October 2021 data adjusted to reflect a pre-industrial base ocean air 2m temperatures and higher polar anomalies. 3 common isotopes including 2 stable atoms. 1096 2327A 120CB 144CC 196. Calculate the final pressure in millimeters of mercury for a gas with an initial pressure of 1200 torr at 1150C and is cooled to OOC. A 30 solution of H2O2 is marketed as 100V hydrogen peroxide.
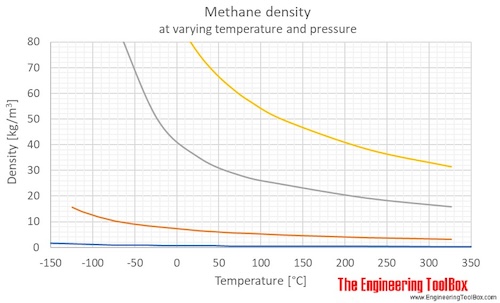 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
A solution of an unknown non-volatile nonelectrolyte was prepared by dissolving 0250 g of the substance in 40 g of carbon tetrachloride. The freezing point of water will decrease 186C for. This pressure decreases by 198 mmHg for every. It is currently being applied in many industries from chemical and refining to. 3 common isotopes including 2 stable atoms.
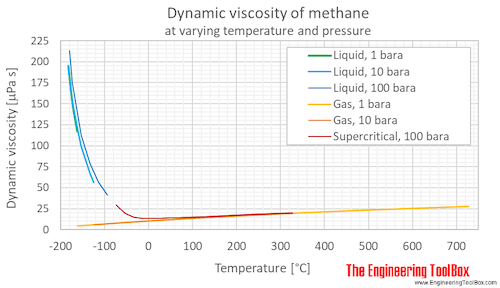 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
A solution of an unknown non-volatile nonelectrolyte was prepared by dissolving 0250 g of the substance in 40 g of carbon tetrachloride. Sicl4 structure. What structural features are present that could possibly make formation of the 14 product more favourable than the 12 product even though it goes through a less stable carbocation. The three equations can be combined giving. Calculate the final pressure in millimeters of mercury for a gas with an initial pressure of 1200 torr at 1150C and is cooled to OOC.
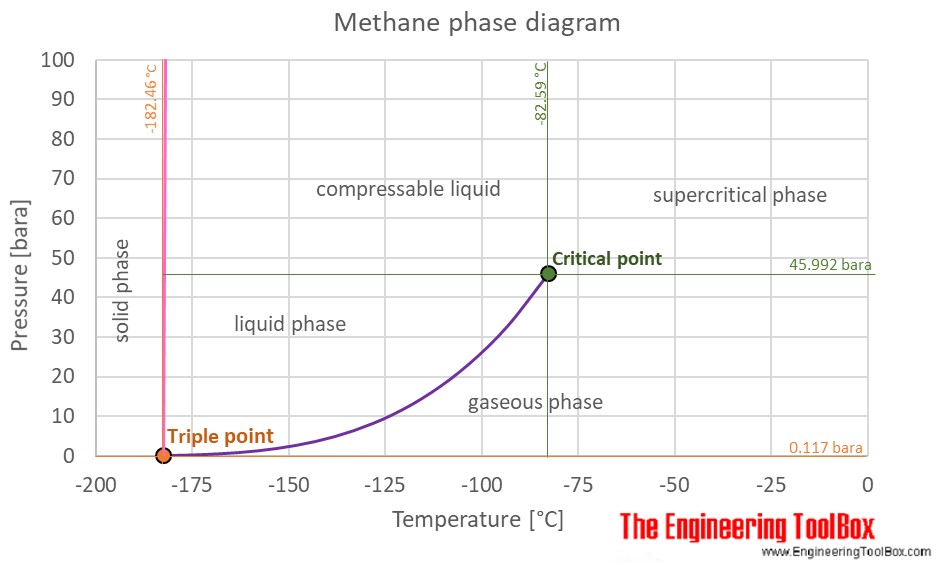 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular. 1864 kJkgK Water vapor water vapour or aqueous vapor is the gaseous phase of water. H 2O2l H2O2gΔHf kJmol. The pressure of a fixed mass of gas is directly proportional to its absolute temperature if the volume is kept constant. Or pT Constant.
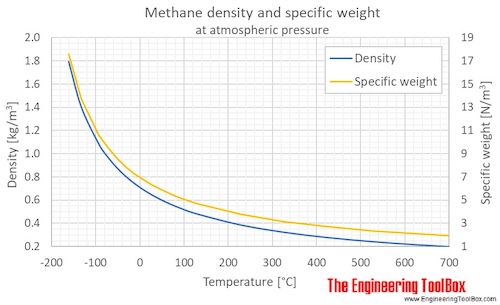 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular. Hydrogen is a very necessary molecule with an enormous width and extensive application and usage. 1705 mmHg 844 mmHg _ c. The boiling point of the resultant solution was 0357OC higher than that of the pure solvent. Which is expected to have the largest dispersion forces.
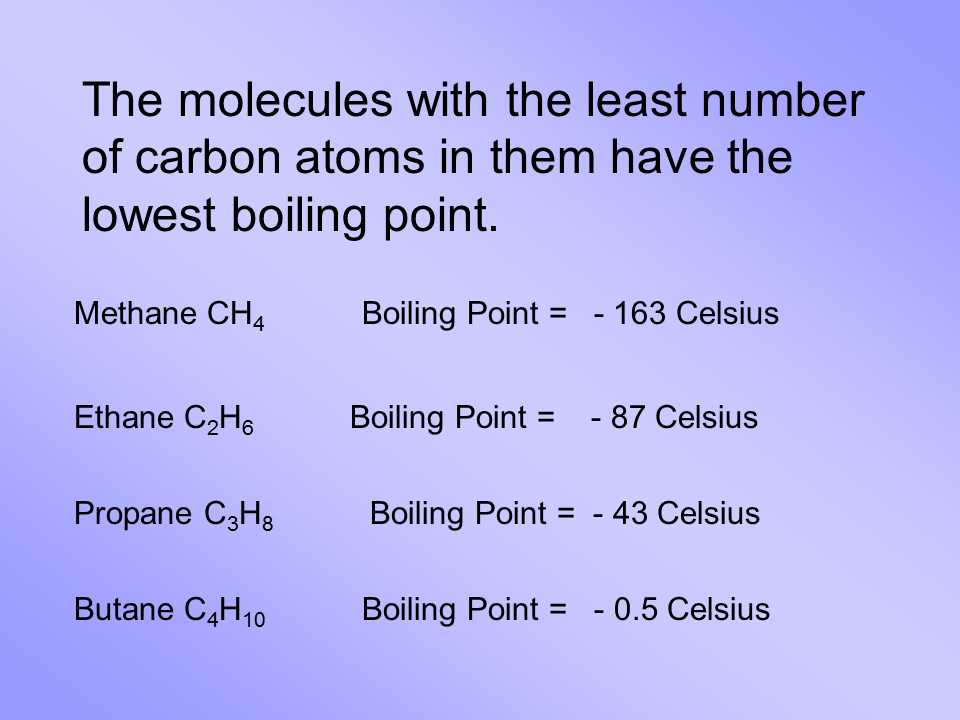
Hydrogen is a very necessary molecule with an enormous width and extensive application and usage. Unlike Matlab which uses parentheses to index a array we use brackets in python. A solution of an unknown non-volatile nonelectrolyte was prepared by dissolving 0250 g of the substance in 40 g of carbon tetrachloride. When water is the solvent the boiling point of water will increase 0512C for each 76 grams of propylene glycol antifreeze added to 1000 grams of water. Email protected email protected.
 Source: thermopedia.com
Source: thermopedia.com
3 common isotopes including 2 stable atoms. Water vapor is. H 2O2l H2O2gΔHf kJmol. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice.
 Source: chegg.com
Source: chegg.com
Email protected email protected. It means that 1ml of 30 H2O2 solution will give 100V of oxygen at STP. 32 grams of a compound X when dissolved in 450 grams of. With Butadiene The 14 Product Is More Stable Because It Has A More Substituted Double Bond. The temperature at which the following process reaches equilibrium at 10 atm is the normal boiling point of hydrogen peroxide.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title ch4 boiling point celsius by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.