Ch2cl2 melting point
Home » datasheet » Ch2cl2 melting pointCh2cl2 melting point
Ch2cl2 Melting Point. CH2Cl2 Lewis structure. The physical properties of CH2Cl2 the compound from the molecule bonds formed are the following. Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp. The most commonly used recrystallization solvents are presented in the following table.
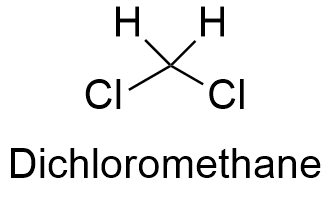 Dichloromethane Formula From softschools.com
Dichloromethane Formula From softschools.com
The American Conference of Governmental Industrial Hygienists recommends. Fluorine is most electronegative then oxygen then nitrogen so bonds between NH3 and NH3 NH3 and H2O H2O and H2O H2O and HF. Why is the melting point of XeF4 higher than that of PF3 even though PF3 is polar but XeF4 isnt. Extract the solution three times with 30-mL portions of dichloromethane CH2Cl2. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule. 118 20 degrees Celsius.
H-H or R3Si-H it goes via a concerted process and they end up cis.
Learning outcomes Students should be able to. What is Hydrochloric Acid. Its boiling point is at 3960C and its melting point is -9760C. Molecular FormulaCH2Cl2 Molecular Weight8493 Section 10 - Stability and Reactivity Chemical Stability. The American Conference of Governmental Industrial Hygienists recommends. 1322 at 68 F USCG 1999 Boiling Point.

440 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air. And a melting point of -967 C. Manual Embedding Cassette Storage. Hydrochloric acid HCL is a clear colourless and pungent solution created by dissolving hydrogen chloride gas in water. Intermolecular Forces As the number of carbons increases in a series of fatty acids the melting point increases.

If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3. Can anyone show me the structure of XeF4 XeF6 XeOF2 mnimgs. 12 NTP 1992 Upper Explosive Limit UEL. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule. Its boiling point is at 3960C and its melting point is -9760C.

118 20 degrees Celsius. 2Describe the alkanes as a homologous series of saturated hydrocarbons with the general formula CnH2n2. 440 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air. Ethanol C2H5 OH will have a greater viscosity than ethylene. Name formula melting point lauric acid C11 H23 COOH 44 C myristic acid C13 H27 COOH 58 C palmitic acid C15 H31 COOH 63 C stearic acid C17 H35 COOH 70 C.
 Source: acs.org
Source: acs.org
Natural sources of dichloromethane include oceanic sources macroalgae wetlands and volcanoes. 2Describe the alkanes as a homologous series of saturated hydrocarbons with the general formula CnH2n2. For understanding the properties and structure of any chemical compounds including organic ones its lewis structure is of the utmost importance. Which of the following will have the highest boiling point. There is a sharp distinction between ionic and covalent bonds when the geometric arrangements of atoms in.
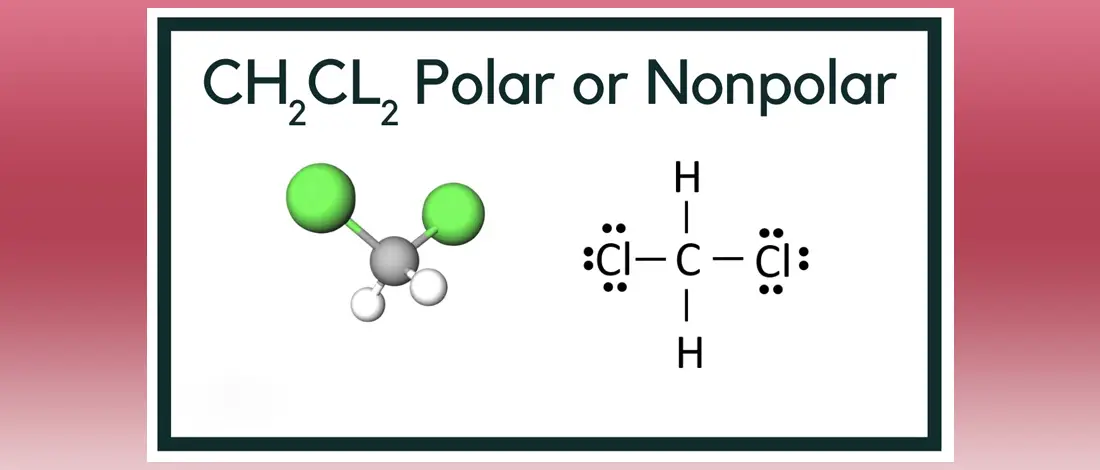 Source: wellcometreeoflife.org
Source: wellcometreeoflife.org
The American Conference of Governmental Industrial Hygienists recommends. 440 mm Hg at 77 F NTP 1992 Vapor Density Relative to Air. Extract the solution three times with 30-mL portions of dichloromethane CH2Cl2. The American Conference of Governmental Industrial Hygienists recommends. Do not get dichloromethane on your hands.
 Source: softschools.com
Source: softschools.com
If you place CH2Cl2 and CHCl3 on a cartesian diagram so that the overall dipole would point to a value of -Y straight down traditionally then the C-Cl bonds have larger -Y values for CH2Cl2 than for CHCl3. And a melting point of -967 C. Methylene chloride is highly volatile boiling point 398C and inert in polyurethane-forming mixtures. 1322 at 68 F USCG 1999 Boiling Point. Embedding Cassette Storage Devices.
2 3 Steps to Determine if a. Manual Embedding Cassette Storage. Stable at room temperature in closed containers under normal storage and handling conditions. This is because as the number of carbons increases the chains get longer. Why is the melting point of XeF4 higher than that of PF3 even though PF3 is polar but XeF4 isnt.
 Source: fishersci.no
Source: fishersci.no
Most pure organic compounds melt over a narrow temperature range of 1-2 C. The most commonly used recrystallization solvents are presented in the following table. Both nonpolar - low melting point low boiling point low water solubility e. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule. Jul 13 2011 Therefore the boiling point is defined for a liquid state while the melting point is defined for a solid state.
 Source: techiescientist.com
Source: techiescientist.com
Accordingly the concentration of methylene chloride in the air inside a foam plant must be kept low. And a melting point of -967 C. 3Draw the structures of branched and unbranched alkanes C1 to C4 and name the unbranched alkanes C1 to C4. However the majority of dichloromethane in the environment is. CH2Cl2 has a dipole moment of 160 D and a boiling point of 40 C.
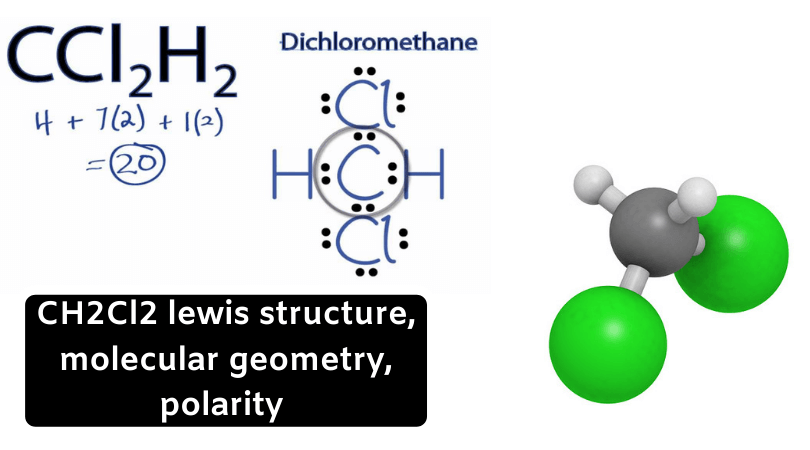 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Extract the solution three times with 30-mL portions of dichloromethane CH2Cl2. 3Draw the structures of branched and unbranched alkanes C1 to C4 and name the unbranched alkanes C1 to C4. The presence of a soluble impurity almost always causes a decrease in the melting point expected for the pure compound and a broadening of the. Thus total seven compounds have non zero dipole moment. Do not get dichloromethane on your hands.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ch2cl2 melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.