Ch2cl2 boiling point
Home » datasheet » Ch2cl2 boiling pointCh2cl2 boiling point
Ch2cl2 Boiling Point. 005 m NaCl D. CH2Cl2 is a similar compound having polar nature as CH2F2. It has a density of 13226gcm3 with a molecular weight of 8496gmol. And if you wanted the best meth you had to come this way you had to come to me.
The C-H bond is less polar than the C-F bond. To start a 150 mL beaker containing 50 mL deionized water and 2 boiling stones was prepared to dissolve 20 grams of sodium carbonate to react with the gallic acid in tea. Which of the following has dipole-dipole attractions. Learning outcomes Students should be able to. The molecular weights of CO HF and Ne are roughly the same. These alcohols form hydrogen bond.
Polar and nonpolar molecules differ significantly.
Which of the following has dipole-dipole attractions. For detailed information regarding factors affecting polarity in such a compound you must refer to the polarity of CH2Cl2. Ethanol C2H5 OH will have a greater viscosity than ethylene. Accordingly the concentration of methylene chloride in the air inside a foam plant must be kept low. 005 m CaCl2 C. According to the VSEPR theory the CHCl3 molecule possesses tetrahedral molecular geometry.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The C-H bond is less polar than the C-F bond. And if you wanted the best meth you had to come this way you had to come to me. Both nonpolar - low melting point low boiling point low water solubility e. Learning outcomes Students should be able to. The chemical compounds low boiling point allows the chemical to function in a heat engine that can extract mechanical energy from small temperature differences.
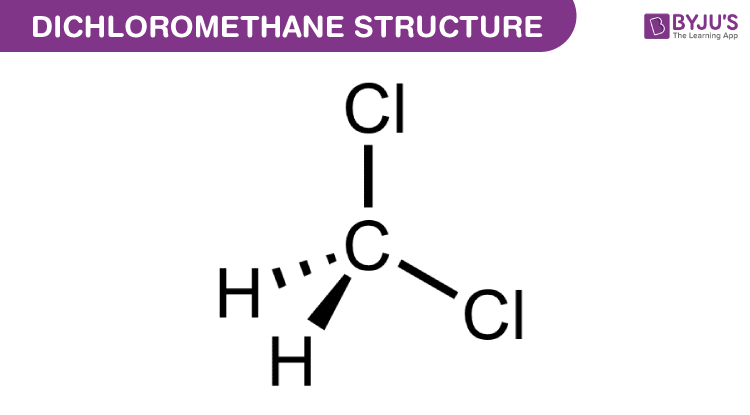 Source: byjus.com
Source: byjus.com
Methylene chloride is highly volatile boiling point 398C and inert in polyurethane-forming mixtures. 012 m C2H4OH2 ethylene glycol A. So the organic layer Compounds were trying to separate insoluble solvent. And a melting point of -967 C. And if you wanted the best meth you had to come this way you had to come to me.
 Source: acs.org
Source: acs.org
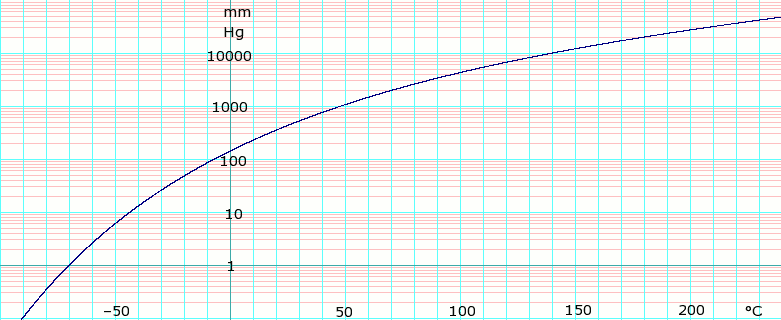
For detailed information regarding factors affecting polarity in such a compound you must refer to the polarity of CH2Cl2. Sodium fluoride NaF acetylene CH and formaldehyde CHO Rank from highest to lowest bolling. So the organic layer Compounds were trying to separate insoluble solvent. Hydrochloric acid HCL is a clear colourless and pungent solution created by dissolving hydrogen chloride gas in water. 1322 at 68 F USCG 1999 Boiling Point.
 Source: fishersci.no
Source: fishersci.no
Organic compounds with one polar functional group and a low number of carbon atoms such as methanol ethanol and n-propanol are highly soluble miscible in water. The beaker was allowed to heat until the water. 19 NTP 1992 Autoignition Temperature. That is one that is held together by stronger dipole forces will have a higher boiling point since it takes more energy to break these intermolecular forces Part B Rank the following compounds in order of decreasing boling point. It is widely used as a solvent in chemistry laboratories.

2 3 Steps to Determine if a. Sodium fluoride NaF acetylene CH and formaldehyde CHO Rank from highest to lowest bolling. Methylene chloride is highly volatile boiling point 398C and inert in polyurethane-forming mixtures. However methylene chloride is a suspected carcinogen and has other deleterious effects on workers exposed to it. Lower Explosive Limit LEL.

Methylene chloride is highly volatile boiling point 398C and inert in polyurethane-forming mixtures. The boiling point of H 2 should be the lowest because it is nonpolar and has the lowest molecular weight. An example of a DCM heat engine is the drinking bird. Thus total seven compounds have non zero dipole moment. And a melting point of -967 C.

Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp. The most commonly used recrystallization solvents are presented in the following table. 1Describe a homologous series and its general characteristics. 005 m C12H22O11 sucrose B. Extract the solution three times with 30-mL portions of dichloromethane CH2Cl2.
 Source: techiescientist.com
Source: techiescientist.com
1036 F at. And if you wanted the best meth you had to come this way you had to come to me. It is a by-product of chlorine manufacture along with sodium hypochlorite. The organic layer also contains a solvent CH2Cl2 or ether that is insoluble in water. The physical properties of CH2Cl2 the compound from the molecule bonds formed are the following.
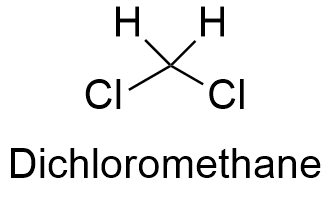 Source: softschools.com
Source: softschools.com
Sodium fluoride NaF acetylene CH and formaldehyde CHO Rank from highest to lowest bolling. State Config State description. ICl is a polar molecule and Br2 is a non-polar molecule. The boiling point of H 2 should be the lowest because it is nonpolar and has the lowest molecular weight. It results in the gaseous nature of difluoromethane with a boiling point of -51C at standard temperature and pressure.

The C-H bond is less polar than the C-F bond. It is widely used as a solvent in chemistry laboratories. 005 m CaCl2 C. If heated the solvent would quickly evaporate due to low boiling point of methylene chloride 2. Polar and nonpolar molecules differ significantly.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title ch2cl2 boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.