Carbon tetrafluoride melting point
Home » datasheet » Carbon tetrafluoride melting pointCarbon tetrafluoride melting point
Carbon Tetrafluoride Melting Point. The greater radii of the larger ions results in smaller lattice energies leading to lower melting points. The melting and boiling point of silicon tetrafluoride is -950 C and -903 C and hence it exists as a gas at room temperature. Elemental carbon is an inert substance insoluble in water diluted acids and bases as well as organic solvents. Several other methods can be used for preparing the element.
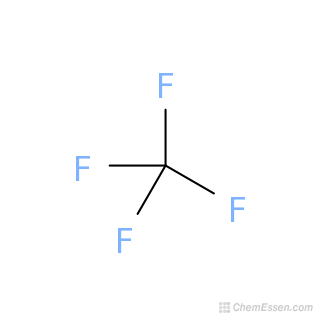 Carbon Tetrafluoride Structure Cf4 Over 100 Million Chemical Compounds Mol Instincts From molinstincts.com
Carbon Tetrafluoride Structure Cf4 Over 100 Million Chemical Compounds Mol Instincts From molinstincts.com
It is a potent greenhouse gas. Amorphous silicon can be prepared as a brown powder which can be easily melted or vaporized. Which of the following correctly explains this trend. A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. The replacement interval for the spark plugs was 112500 kilometres. Silicon is prepared commercially by heating silica and carbon in an electric furnace using carbon electrodes.
The EJ204 engine had an ignition knock control facility with fuzzy.
1119 C 1694 F boiling point. If this solution is ideal its total vapor pressure at 20C is. 1080 C 1624 F density 1 atm 0 C 32 F 5887 glitre 0078 ouncegallon oxidation states. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. At high temperatures it binds with oxygen to form carbon monoxide or dioxide. With hot oxidizing agents like nitric acid and potassium nitrate metilic acid C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The Czochralski process is commonly used to produce single crystals of silicon used for solid-state or semiconductor devices. By contrast the ionic solid NaCl has a melting point of 800C. The Czochralski process is commonly used to produce single crystals of silicon used for solid-state or semiconductor devices. The vapor pressure of pure benzene at this temperature is 7461 mmHg and its density is 087865 gcm3. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable.
 Source: globalsources.com
Source: globalsources.com
LiF NaCl KBr CsI. The EJ204 engine had an ignition coil for each cylinder that was positioned directly above the platinum-tipped spark plug. Usually occurs between non-metals. By contrast the ionic solid NaCl has a melting point of 800C. The EJ204 engine had an ignition knock control facility with fuzzy.
 Source: molinstincts.com
Source: molinstincts.com
1 Production of R11 or CFC-11 was halted by the clean air act on January 1 1996 2 Production of R12 or CFC-12 Dichlorodifluoromethane was halted by the clean air act on January 1 1996 3 R22 or HCFC-22 is a single component HCFC refrigerant with low ozone depletion potential. It is a potent greenhouse gas. Carbo coal is a chemical element with the symbol C and atomic number 6. 0 2 4 6 8. Drawing the lewis structure for hcn.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This has a high melting point 800 ºC and dissolves in water to to give a conducting solution. The EJ204 engine had an ignition knock control facility with fuzzy. Amorphous silicon can be prepared as a brown powder which can be easily melted or vaporized. Zirconium powder dry appears as a gray amorphous powder. 1 Production of R11 or CFC-11 was halted by the clean air act on January 1 1996 2 Production of R12 or CFC-12 Dichlorodifluoromethane was halted by the clean air act on January 1 1996 3 R22 or HCFC-22 is a single component HCFC refrigerant with low ozone depletion potential.
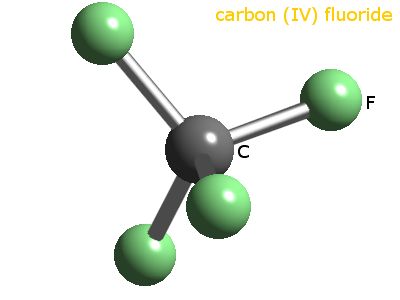 Source: webelements.com
Source: webelements.com
It is nonflammable and soluble in chloroform and benzene. It has a molar mass of 880043 gmol. Amorphous silicon can be prepared as a brown powder which can be easily melted or vaporized. Prydz and Goodwin 1972. 1080 C 1624 F density 1 atm 0 C 32 F 5887 glitre 0078 ouncegallon oxidation states.
 Source: yanxachem.com
Source: yanxachem.com
LiF NaCl KBr CsI. The blends should be diluted with. A solution is 4000 by volume benzene C6H6 in carbon tetrachloride at 20C. 1 Production of R11 or CFC-11 was halted by the clean air act on January 1 1996 2 Production of R12 or CFC-12 Dichlorodifluoromethane was halted by the clean air act on January 1 1996 3 R22 or HCFC-22 is a single component HCFC refrigerant with low ozone depletion potential. The Czochralski process is commonly used to produce single crystals of silicon used for solid-state or semiconductor devices.
Carbo coal is a chemical element with the symbol C and atomic number 6. By contrast the ionic solid NaCl has a melting point of 800C. The greater radii of the larger ions results in smaller lattice energies leading to lower melting points. Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a radionuclide. The Czochralski process is commonly used to produce single crystals of silicon used for solid-state or semiconductor devices.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It belongs to group 14 of the periodic table. 0 2 4 6 8. Prydz and Goodwin 1972. The EJ204 engine had an ignition coil for each cylinder that was positioned directly above the platinum-tipped spark plug. Amorphous silicon can be prepared as a brown powder which can be easily melted or vaporized.
 Source: en.wikipedia.org
Source: en.wikipedia.org
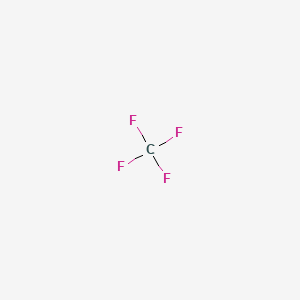
Ignitable by static electricity. Tetrafluoromethane also known as carbon tetrafluoride or R-14 is the simplest perfluorocarbon C F 4As its IUPAC name indicates tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methaneIt can also be classified as a haloalkane or halomethaneTetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. 1 Production of R11 or CFC-11 was halted by the clean air act on January 1 1996 2 Production of R12 or CFC-12 Dichlorodifluoromethane was halted by the clean air act on January 1 1996 3 R22 or HCFC-22 is a single component HCFC refrigerant with low ozone depletion potential. Xenon occurs in slight traces in gases within Earth and is present to an extent of about 00000086 percent or about 1 part in 10 million by. If the melting point is too high additional heating may be required to prevent freezing.
 Source: wikiwand.com
Source: wikiwand.com
The vapor pressure of pure carbon tetrachloride is 9132 mmHg and its density is 15940 gcm3. LiF NaCl KBr CsI. The Czochralski process is commonly used to produce single crystals of silicon used for solid-state or semiconductor devices. Zirconium powder dry appears as a gray amorphous powder. Amorphous silicon can be prepared as a brown powder which can be easily melted or vaporized.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title carbon tetrafluoride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.