Carbon tetrachloride melting point
Home » datasheet » Carbon tetrachloride melting pointCarbon tetrachloride melting point
Carbon Tetrachloride Melting Point. Therefore the polyethylene formed is in the solid phase. Halogen and inert gases melting and boiling points. It is practically not flammable at lower temperatures. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid.
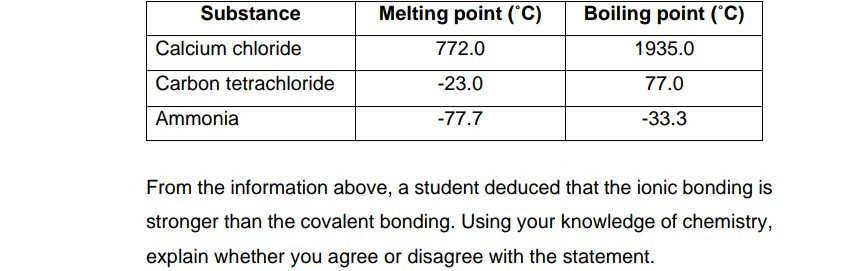 Solved Substance Melting Point C 772 0 Boiling Point C Chegg Com From chegg.com
Solved Substance Melting Point C 772 0 Boiling Point C Chegg Com From chegg.com
A domesticated carnivorous mammal Canis familiaris syn. Because of their high melting point and poor solubility in most solvents Kevlar and Nomex proved to be a challenge but this was eventually solved. The melting and freezing point changes with pressure but normally they are given at 1 atm. Energy of second ionisation. Carl Wilhelm Scheele in 1774. At C10 level the elements present will be told to you but read up the method.
The melting and freezing point changes with pressure but normally they are given at 1 atm.
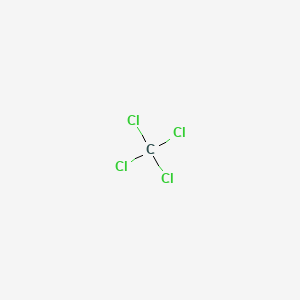
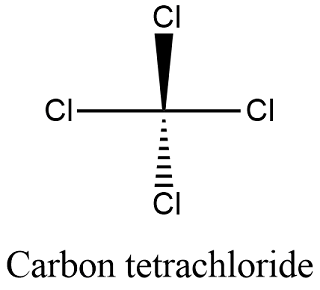
Carbon tetrachloride also known by many other names such as tetrachloromethane also recognised by the IUPAC carbon tet in the cleaning industry Halon-104 in firefighting and Refrigerant-10 in HVACR is an organic compound with the chemical formula CCl 4It is a colourless liquid with a sweet smell that can be detected at low levels. 28085 State at 20C. The melting and freezing point changes with pressure but normally they are given at 1 atm. Its melting point is -23C. Boiling point of water. Energy of third ionisation.
 Source: slideplayer.com
Source: slideplayer.com
It belongs to group 14 of the periodic table. Energy of first ionisation. Phase at Room Temperature. Energy of third ionisation. It is practically not flammable at lower temperatures.
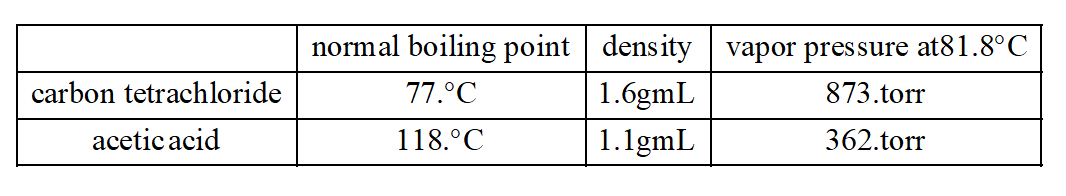 Source: bartleby.com
Source: bartleby.com
Three isotopes occur naturally 12 C and 13 C being stable while 14 C is a radionuclide. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. Carbon makes up only about 0025 percent of Earths crust. Carl Wilhelm Scheele in 1774. P block contains the highest melting points element carbon and lowest melting point element of the periodic table helium.
Source: chemspider.com
Phase at Room Temperature. In such a bond there is a charge separation with one atom being slightly more positive and the other more negative ie the bond will produce a dipole moment. Electronic shell Ne 3s 2 3p 5. The slurry solution is passed to a catalyst decomposition bed where the catalyst is deactivated. 100 C 212 F Boiling point of water in Kelvin.
Source: en.wikipedia.org
CCl4 is not very soluble in water. Halogen and inert gases melting and boiling points. This type of polymerization is called slurry polymerization or suspension polymerization. Many carbon compounds are essential for life as we know it. Carbo coal is a chemical element with the symbol C and atomic number 6.
 Source: suvidhinathlab.com
Source: suvidhinathlab.com
Because of their high melting point and poor solubility in most solvents Kevlar and Nomex proved to be a challenge but this was eventually solved. In such a bond there is a charge separation with one atom being slightly more positive and the other more negative ie the bond will produce a dipole moment. ChemSpider is a free chemical structure database Glossary. Melting Point of Pure Titanium. Some of the most common carbon compounds are.
 Source: thoughtco.com
Source: thoughtco.com
Carl Wilhelm Scheele in 1774. For example the molecule carbon tetrachloride is a non-polar covalent molecule CCl 4. 3265C 5909F 3538 K Block. Phase at Room Temperature. It belongs to group 14 of the periodic table.
 Source: chegg.com
Source: chegg.com
Another group of polymers characterized by a high degree of cross-linking resist deformation and solution once their final morphology is achieved. Such polymers are usually prepared in molds that. Gases liquids or solids. 1414C 2577F 1687 K Period 3 Boiling point. CCl4 is not very soluble in water.
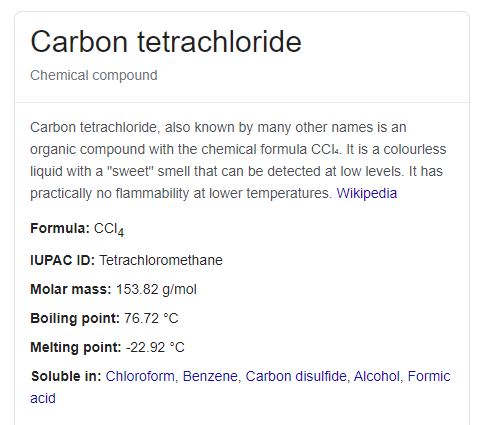 Source: waterfilteradvisor.com
Source: waterfilteradvisor.com
The solubility of the unknown in. In general melting is a phase change of a substance from the solid to the liquid phase. Standard potential - 136 V. It belongs to group 14 of the periodic table. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed.
The catalyst is not completely used in the polymerization process. 32 14 57 52 11 Emergency Number. -1958 C -3204 F Boiling point of liquid helium. 3265C 5909F 3538 K Block. The melting and freezing point changes with pressure but normally they are given at 1 atm.
 Source: softschools.com
Source: softschools.com
Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. It belongs to group 14 of the periodic table. Carbon tetrachloride is a manufactured chemical that does not occur naturally. Thermodynamics - Effects of work heat and energy on systems. The melting and freezing point changes with pressure but normally they are given at 1 atm.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title carbon tetrachloride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.