Carbon tetrachloride boiling point
Home » datasheet » Carbon tetrachloride boiling pointCarbon tetrachloride boiling point
Carbon Tetrachloride Boiling Point. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable. It has a Tetragonal coordination geometry.
 Solved Given The Graph Right What Is The Boiling Point Of Chegg Com From chegg.com
Solved Given The Graph Right What Is The Boiling Point Of Chegg Com From chegg.com
This section is from the book Distillation Principles And Processes by Sydney Young. Phase at Room Temperature. All boiling points below are normalatmospheric boiling points. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Melting point C cyanogen CN 2. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable.
For example the molecule carbon tetrachloride is a non-polar covalent molecule CCl 4.
Carbon makes up only about 0025 percent of Earths crust. The solubility of the unknown in. By weight in mixture. Carbon tetrachloride is a manufactured chemical that does not occur naturally. Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. 154 Inorganic carbon-nitrogen compounds.
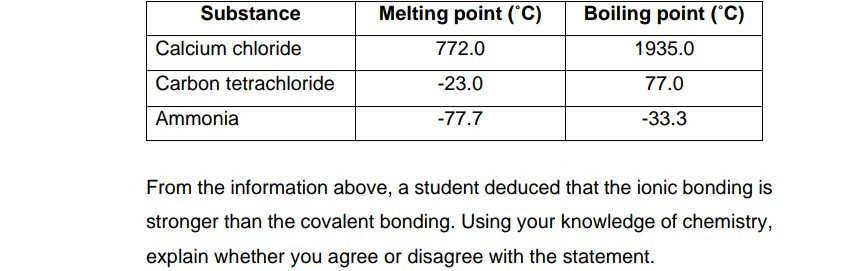 Source: chegg.com
Source: chegg.com
Determine the boiling point or melting point. 154 Inorganic carbon-nitrogen compounds. At C10 level the elements present will be told to you but read up the method. When a solute is added to the solvent some of the solute molecules occupy the space near the surface of the liquid as shown in the figure below. In the manufacturing of various chemicals.
 Source: clutchprep.com
Source: clutchprep.com
Some of the most common carbon compounds are. Distillation is recommended in the case of liquids see Appendix 3. CCl4 has a boiling point of 7672 C and a melting point of 2292 C. It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO.
 Source: schoolbag.info
Source: schoolbag.info
It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds. It is also called carbon chloride methane tetrachloride perchloromethane tetrachloroethane or benziformCarbon tetrachloride is most often found in the air as a. Whats in a name. Free Books Science Distillation Principles And Processes List Of Known Azeotropic Mixtures. Some of the most common carbon compounds are.
 Source: thoughtco.com
Source: thoughtco.com
Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable. It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds. Hydrogen and another non-metal chemically combines through. 154 Inorganic carbon-nitrogen compounds. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
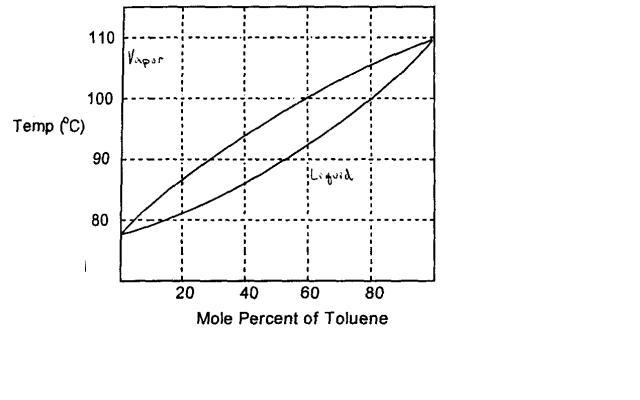 Source: chegg.com
Source: chegg.com
Also available from Amazon. Hydrogen and another non-metal chemically combines through. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. Determine the boiling point or melting point. Acetic acid anhydride CH 3.
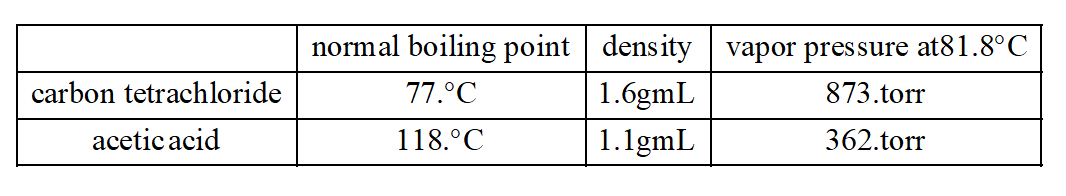 Source: bartleby.com
Source: bartleby.com
It is nonmetallic and tetravalentmaking four electrons available to form covalent chemical bonds. Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. Carbon makes up only about 0025 percent of Earths crust. All boiling points below are normalatmospheric boiling points. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions.
 Source: slideplayer.com
Source: slideplayer.com
It is used as a fire extinguisher. It is used as a solvent for halogenation and as an agricultural fumigant. It has a Tetragonal coordination geometry. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. CCl4 is not very soluble in water.
 Source: chegg.com
Source: chegg.com
At a temperature of 25 degrees Celsius the solubility of carbon tetrachloride in water is only 1 gram per litre approx. Also available from Amazon. Carbon makes up only about 0025 percent of Earths crust. Carbo coal is a chemical element with the symbol C and atomic number 6. Carbon tetrachloride is a manufactured chemical that does not occur naturally.
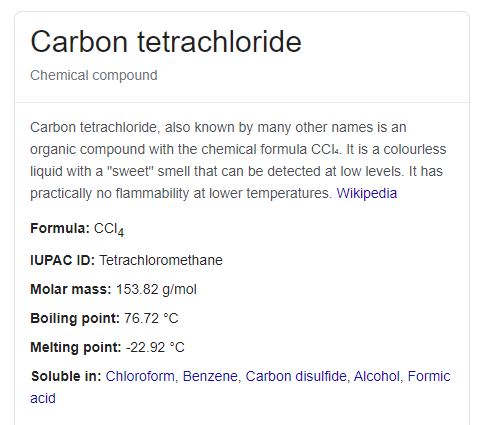 Source: waterfilteradvisor.com
Source: waterfilteradvisor.com
CN 2 H 2. CN 2 H 2. Many carbon compounds are essential for life as we know it. Also available from Amazon. Distillation is recommended in the case of liquids see Appendix 3.
Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Carbo coal is a chemical element with the symbol C and atomic number 6. Binary Azeotropic Mixtures - Minimum Boiling Point Substances in mixture. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. CCl4 is not very soluble in water.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title carbon tetrachloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.