Cao melting point
Home » datasheet » Cao melting pointCao melting point
Cao Melting Point. The chemical formula of calcium oxide is CaO. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium. To properly assign this feature to melting ILZIF-8 was heated at 387 and 390 C under nitrogen slightly above T m defined as the offset temperature of the melting peak for 30 and 40 min. Which of the following will require the greatest energy input to separate the ions.
 Veryyyy Confused I Thought That Melting Point Bp Fp Are All Colligative Properties Meaning Independent Of The Identity Of The Compound But Dependent On Mass Or Number Of Particles Why Would Cao Have From reddit.com
Veryyyy Confused I Thought That Melting Point Bp Fp Are All Colligative Properties Meaning Independent Of The Identity Of The Compound But Dependent On Mass Or Number Of Particles Why Would Cao Have From reddit.com
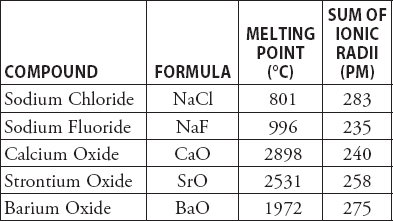
The water or vapor content in air varies. Calcium oxide is more normally made by heating calcium carbonate. Quick lime is an amorphous white solid with a high melting point of 2600. Use atomic or molecular properties to explain why calcium oxide has a much higher melting point 2580 degrees Celsius than potassium fluoride 858 degrees Celsius. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. Of both historical and etymological interest is the use of lime as an illuminant in stage lighting for some years before the advent of electricity 1850-1880.
328 ppm 1980 aprox.
Commercial vacuum induction melting VIM was developed in the early 1950s having been stimulated by the need to. Platinum-iridium bar at melting point of ice atmospheric pressure supported by two rollers 7th CGPM 1927. Properties of Calcium Oxide. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. 4325 Towards a Synthetic Understanding of the Drivers of Exposure and Vulnerability. 320 ppm 1970 aprox.
 Source: researchgate.net
Source: researchgate.net
The element is likely to be a Calcium b Carbon c Silicon d Iron. The Federal response to Hurricane Sandy illustrated the breadth and depth of response necessary from US. Use atomic or molecular properties to explain why calcium oxide has a much higher melting point 2580 degrees Celsius than potassium fluoride 858 degrees Celsius. Calcium reacts with oxygen to give calcium oxide. Soda Lime Glass Melting Point.
 Source: quora.com
Source: quora.com
The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium. The chemical formula of calcium oxide is CaO. We say that such a body melts. Soda-lime glass is composed of 73 SiO 2 15 Na 2 O 7 CaO 4 MgO 1 Al 2 O3. Use atomic or molecular properties to explain why calcium oxide has a much higher melting point 2580 degrees Celsius than potassium fluoride 858 degrees Celsius.
 Source: bartleby.com
Source: bartleby.com
Effects of CaO on the reduction degree of pellets at different stages a reduction degree with adding different ratios of CaO b reduction degree at different stages. Quick lime is an amorphous white solid with a high melting point of 2600. Which of the following will have the highest melting point. However the take up of CO2 is much greater with plant growth. The composition of air is unchanged until elevation of approximately 10000 m.
 Source: scielo.org.za
Source: scielo.org.za
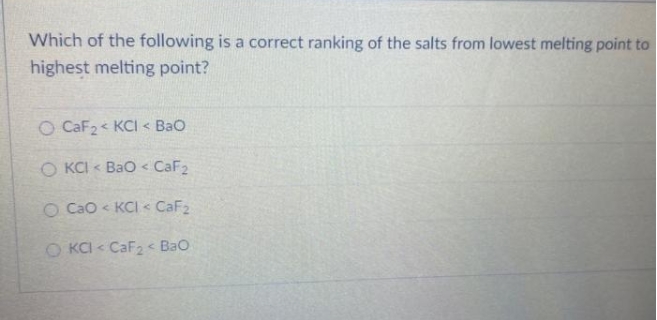
Which of the following requires the lowest melting point. A glass of any kind is a frozen liquid so there is no exact melting point. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O. Security personnel in the wake of. 372 ppm 2010 aprox.
 Source: reddit.com
Source: reddit.com
Forsterite melts at a much higher temperature so further melting cannot take place until the temperature reaches. The water or vapor content in air varies. Vacuum Induction Melting MELTING UNDER VACUUM in an induc-tion-heated crucible is a tried and tested process intheproductionofliquidmetalIthasitsorigins in the middle of the 19th century but the actual technical breakthrough occurred in the second half of the 20th century. A from level 457 sample E313 413 cm depth. Electrolysis of hot molten MgCl 2 affords magnesium as a liquid whih is poured off and chlorine gas.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu
Commercial vacuum induction melting VIM was developed in the early 1950s having been stimulated by the need to. Arrange the three compounds sodium. 165076373 wavelengths of light from a specified transition in krypton-86 11th CGPM 1960. 390 ppm and 2020 aprox. This metal peroxide exists in several hydrates and peroxyhydrates including Na 2 O 2 2H 2 O 2 4H 2 O Na 2 O 2 2H 2 O Na 2 O 2 2H 2 O 2 and Na 2 O 2 8H 2 O.
 Source: crackap.com
Source: crackap.com
4322 Settlement Trends. CaO H 2 O Ca 2 2OH-Mg 2 2OH- MgOH 2. Security personnel in the wake of. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium. 356 ppm 2000 aprox.
 Source: researchgate.net
Source: researchgate.net
Soda-lime glass is composed of 73 SiO 2 15 Na 2 O 7 CaO 4 MgO 1 Al 2 O3. A glass of any kind is a frozen liquid so there is no exact melting point. Onto the shore of Breezy Point New York on Nov. Which of the following requires the lowest melting point. Invented in 1816 this technique involved an oxyhydrogen flame impinging on a cylinder of lime causing.
 Source: steeldata.info
Source: steeldata.info
The limewater turns cloudy as white calcium carbonate is e In terms of attractive forces explain why LiCl has a higher melting point than SCl 2. At this point all that is left in the rock is Forsterite. Invented in 1816 this technique involved an oxyhydrogen flame impinging on a cylinder of lime causing. The water or vapor content in air varies. This can be recovered as magnesium chloride MgCl 2 through reaction with calcium oxide CaO.
 Source: researchgate.net
Source: researchgate.net
Soda-lime glass is composed of 73 SiO 2 15 Na 2 O 7 CaO 4 MgO 1 Al 2 O3. Like other high-melting solids tungsten zirconia carbon lime CaO becomes incandescent when heated to near its mp 2500 C. Calcium reacts with oxygen to give calcium oxide. The Federal response to Hurricane Sandy illustrated the breadth and depth of response necessary from US. To properly assign this feature to melting ILZIF-8 was heated at 387 and 390 C under nitrogen slightly above T m defined as the offset temperature of the melting peak for 30 and 40 min.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title cao melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.