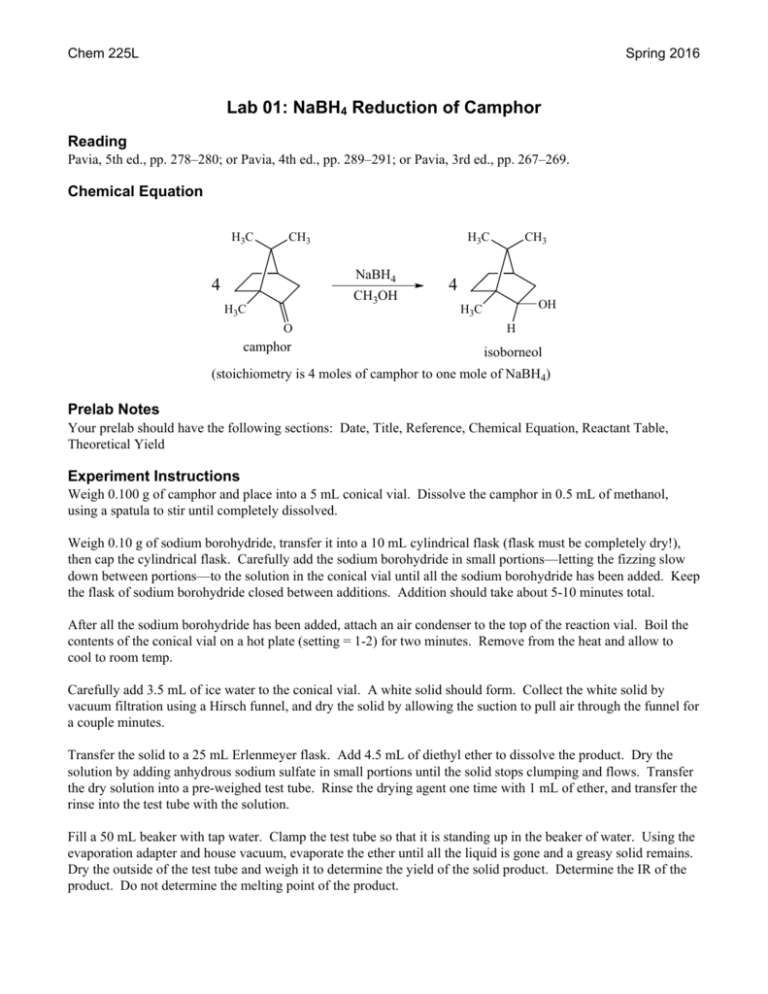
Camphor melting point experiment
Home » datasheet » Camphor melting point experimentCamphor melting point experiment
Camphor Melting Point Experiment. Each isomer will have different physical properties of boiling point melting point period Lesson Isomers pages 717721 BLOCK SCHEDULE LESSON PLAN 22. Whereas burning of wax is a chemical change as carbon dioxide and water are formed when wax is burned. The fish fillets were homogenized and extracted by Soxhlet extraction. A suspension of soil chalk powder and fine sand.
 Values Of Melting Points Of Synthesized Racemic Camphor Derivatives And Download Table From researchgate.net
Values Of Melting Points Of Synthesized Racemic Camphor Derivatives And Download Table From researchgate.net
This is carried out by extracting heat from the liquid. Rusting is a slow process in which the surface of iron is spoiled due to the action of oxygen and water on it. LESSON PLAN 22 - Glencoe explaining the number of isomers observed. When certain low melting point solids are mixed. Distinguishing Between Solutions To prepare - a true solution of common salt sugar and alum. B It is the property of the gas.
Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage.
To determine the melting point of ice. A colloidal of starch and egg albumin and distinguish between these on the basis of - transparency filtration and stability. Each isomer will have different physical properties of boiling point melting point period Lesson Isomers pages 717721 BLOCK SCHEDULE LESSON PLAN 22. Thin Layer Chromatography and Melting Point. B Sulphur reacts with iron to form a black colour compound FeS. One of its main constituents is cetyl palmitate another ester of a fatty acid and a fatty alcohol.
 Source: studocu.com
Source: studocu.com
An analytical method for the determination of UV filter substances in fish tissue has been developed and validated using benzophenone-3 3-4-methylbenzylidene-camphor 2-ethylhexyl-2-cyano-33-diphenyl-2-propenoate and 2-ethylhexyl 3-methoxyphenyl-2-propenoate as target analytes. Pdf Text File. Whereas burning of wax is a chemical change as carbon dioxide and water are formed when wax is burned. Melting point of this alloy 945C Melting point of Pb 327C Melting point of Sn 2319C Melting point of Bi 271C Freezing. To determine the boiling point of water.
 Source: researchgate.net
Source: researchgate.net
If crayons or colored pencils. Hydrogen Balloons Latex 1 Responses. Draw a diagram of the paper chromatography equipment. This was shown by the melting point of the product 1624 to 1633C. One of its main constituents is cetyl palmitate another ester of a fatty acid and a fatty alcohol.
 Source: studylib.net
Source: studylib.net
A FeS is black colour compound. Every pure substance has its own particular melting point and boiling point. D The melting point of sulphur is low. Together a liquid or soft mass know as eutec tic mixture is. Liquid in solid Dental amalgam Mercury liquid Silver solid Solid in solid Brass Zinc solid Copper solid Solutions form between solute and solvent molecules because of similarities between them.
 Source: studylib.net
Source: studylib.net
The fish fillets were homogenized and extracted by Soxhlet extraction. A solution of 0090 g of this compound in 110 g of camphor melts at 1584 C. To determine the boiling point of water. A solution of 0090 g of this compound in 110 g of camphor melts at 1584 C. LESSON PLAN 22 - Glencoe explaining the number of isomers observed.
 Source: chemistry.mcmaster.ca
Source: chemistry.mcmaster.ca
A FeS is black colour compound. But the teacher told him that actual melting point of the solid is text150 circtext C. 86 Compound Camphor Carbon Tetrachloride Ethanol Water Boiling Point 80. Thin Layer Chromatography and Melting Point. Chromatography Simran Sharda CHM 1004 ETX41725 Dr.
 Source: studocu.com
Source: studocu.com
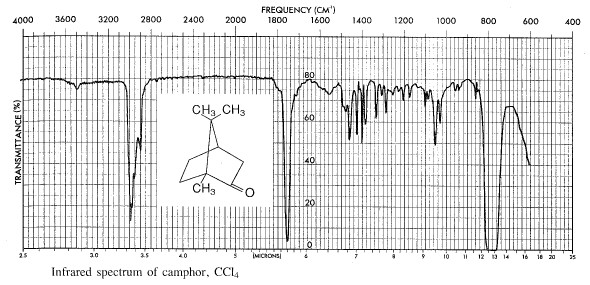
Chromatography Simran Sharda CHM 1004 ETX41725 Dr. K f for camphor is 377 Cm. B It is the property of the gas. As an aromatic hydrocarbon naphthalenes structure consists of a fused pair of benzene rings. The melting point of pure camphor is 1784 C.
Source:
Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. Rusting is a slow process in which the surface of iron is spoiled due to the action of oxygen and water on it. Reason R. ProducedThis occurs due to the lowering of the melting. Ionic compounds in molten form or in solution can conduct electricity while The unit cell edge length is 409.
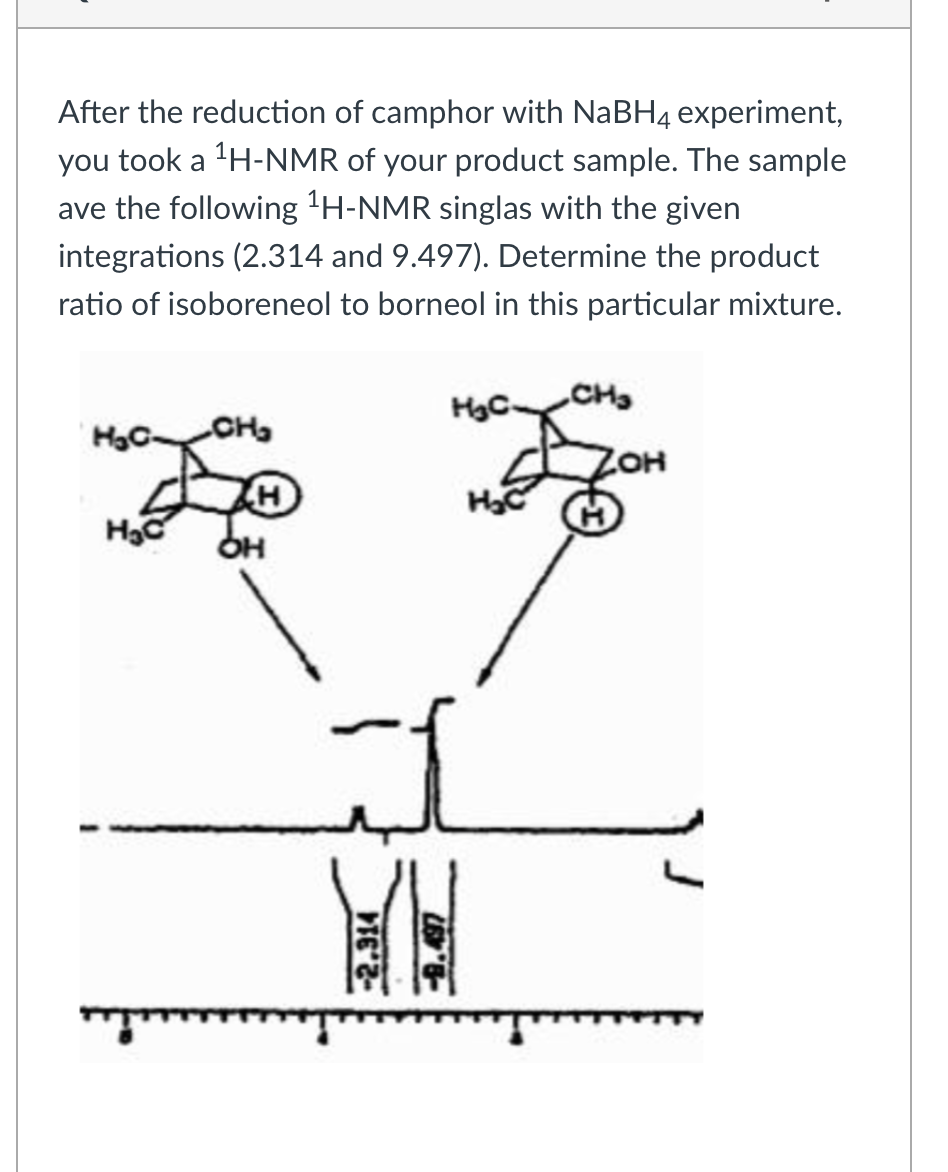
Together a liquid or soft mass know as eutec tic mixture is. Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. Alert regarding hydrogen balloon explosions. Hydrogen Balloons Latex 1 Responses. If crayons or colored pencils.
 Source: studylib.net
Source: studylib.net
Liquid in solid Dental amalgam Mercury liquid Silver solid Solid in solid Brass Zinc solid Copper solid Solutions form between solute and solvent molecules because of similarities between them. Liquid in solid Dental amalgam Mercury liquid Silver solid Solid in solid Brass Zinc solid Copper solid Solutions form between solute and solvent molecules because of similarities between them. When a substance in the liquid state is subjected to cooling heat is. One way to check the purity of the separated liquid is to measure its boiling point. When certain low melting point solids are mixed.
 Source: researchgate.net
Source: researchgate.net
Whereas burning of wax is a chemical change as carbon dioxide and water are formed when wax is burned. The extracts were run through a clean-up. When a substance in the liquid state is subjected to cooling heat is. K f for camphor is 377 Cm. A student is asked to determine what mass of butane needed to burn in order to raise the temperature email protected 00 C to 55 C.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title camphor melting point experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.