Calcium chloride boiling point
Home » datasheet » Calcium chloride boiling pointCalcium chloride boiling point
Calcium Chloride Boiling Point. Calcium does not occur naturally in the free state but compounds of the element are widely distributed. The compound is therefore electrically neutral. Its molecular weight is 11098 gmol and its melting point is 772 C. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid.
 Calcium Chloride From cs.mcgill.ca
Calcium Chloride From cs.mcgill.ca
Sodium chloride is the least expensive option but is less effective because it only dissociates into two ions instead of three. Why group IIA elements melting at higher temperatures than group IA elements. Calcium chloride is shipped in three basic forms. Low calcium intake may also be a risk factor in the development of osteoporosis. Calcium gluconate and calcium lactate are absorbed well by pregnant women. Alkyl halide can be prepared.
Boiling point - the temperature at which a liquid turns into a gas.
Bone serves as an important storage point for calcium as it contains 99 of the total body calcium. The colour of aluminium chloride is white but often it is contaminated with iron trichloride which makes it yellow. Why boiling point of calcium. It can be created by neutralising hydrochloric acid with calcium hydroxide. Chlorocalcite KCaCl 3. It can be noted that the calcium cation holds a charge of magnitude 2 and each chloride anion holds a charge of magnitude -1.
 Source: freechemistryonline.com
Source: freechemistryonline.com
The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected mainly with dry lakes and brines. This is due to the increased strength of the intermolecular forcesfrom London dispersion to dipole-dipole interaction because of the increased polarity. The compounds of. The salt has to dissolve into its ions in order to work. Calcium Carbonate is the carbonic salt of calcium CaCO3.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
It is in a liquid state only at pressures above 25 atm and temperature above 190C. One calcium compound lime calcium oxide CaO was extensively used by the ancients. Wear goggles and a lab coat. Magnesium chloride works down to 5F while calcium chloride works down to -20F. Unlike magnesium calcium is quite difficult to ignite but once lit it burns with a brilliant high-intensity red flame.
 Source: clutchprep.com
Source: clutchprep.com
Magnesium chloride works down to 5F while calcium chloride works down to -20F. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid. Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 Source: sciencedirect.com
Source: sciencedirect.com
Calcium chloride is an inorganic compound a salt with the chemical formula CaCl 2. AlCl3 in a molten state is a poor conductor of electricity. Calcium chloride CaCl2 is a natural compound of calcium and chlorine derived from limestone. Today we obtain calcium through the electrolysis of a fused salt such as calcium chloride. The figure below shows the phase diagram for a pure solvent and how it changes when a.
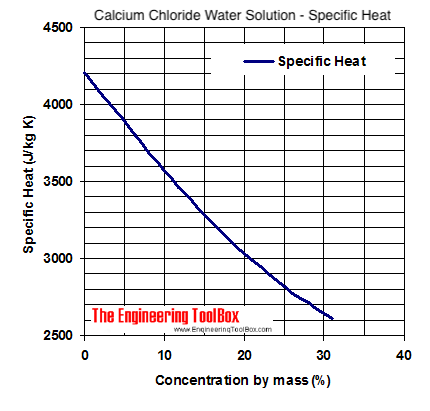 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Low calcium intake may also be a risk factor in the development of osteoporosis. Boiling Point-121 F at 760 mm Hg A constant boiling azeotrope with water containing 2022 hydrogen chloride boils at 227 F EPA 1998 Molecular Weight. Calcium does not occur naturally in the free state but compounds of the element are widely distributed. Melting point - the temperature at which a solid turns into a liquid. Calcium chloride molecules feature two ionic bonds between the single calcium cation and the two chloride anions.
 Source: hydro-land.com
Source: hydro-land.com
Calcium does not occur naturally in the free state but compounds of the element are widely distributed. 823 g100 g at 32 F NTP 1992. During pregnancy lead is released from bones as maternal calcium and is used to help form the bones of the fetus. Colligative Properties of Matter. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid.
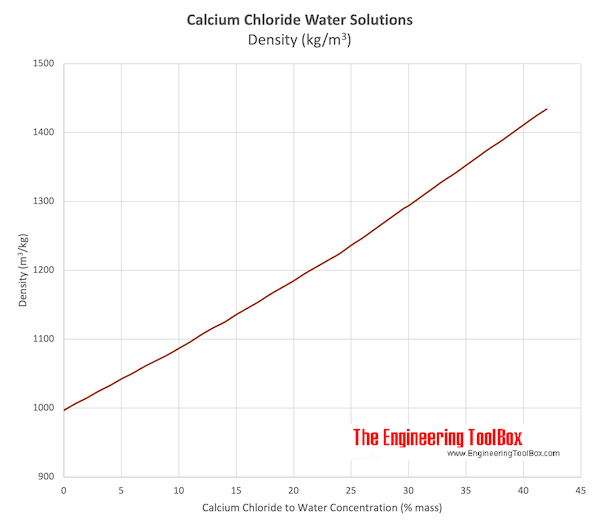 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
During pregnancy lead is released from bones as maternal calcium and is used to help form the bones of the fetus. Freezing point depression is a colligative property of matter. The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected mainly with dry lakes and brines. The figure below shows the phase diagram for a pure solvent and how it changes when a. Lead can also cross the placental barrier exposing the fetus to lead.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
During pregnancy lead is released from bones as maternal calcium and is used to help form the bones of the fetus. Low calcium intake may also be a risk factor in the development of osteoporosis. Calcium chloride is shipped in three basic forms. Metallic lattices of group ii metals are much stronger than group i metals because group ii elements give two electrons to the lattice. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass.
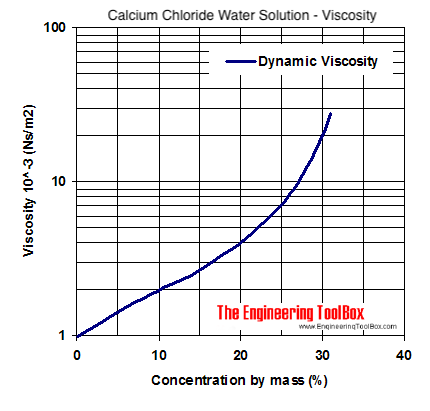 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Colligative Properties of Matter. Calcium ChlorideDihydrate Created by Global Safety Management Inc. The molecules in the following show some of the example of alkyl halides. It is a white colored crystalline solid at room temperature and it is highly soluble in water. Calcium chloride molecules feature two ionic bonds between the single calcium cation and the two chloride anions.
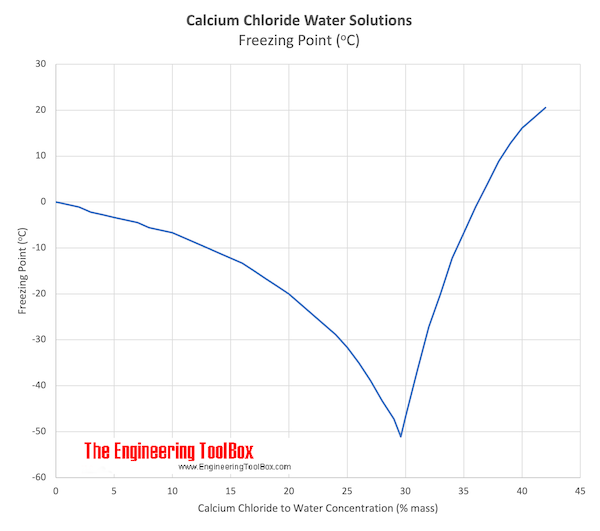 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The colour of aluminium chloride is white but often it is contaminated with iron trichloride which makes it yellow. Aluminium chloride has a very low melting and boiling point. 823 g100 g at 32 F NTP 1992. 1484 C 2703 F specific gravity. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calcium chloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.