Calcium carbonate melting point
Home » datasheet » Calcium carbonate melting pointCalcium carbonate melting point
Calcium Carbonate Melting Point. The limestone is added. This means that calcium needs more than 14 times of needed heat to boil water. Download Product Safety Card. The general method of ester preparation can be.
 Calcium Carbonate Wikipedia From en.wikipedia.org
Calcium Carbonate Wikipedia From en.wikipedia.org
1484C 2703F 1757 K. It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of eggshells snail shells seashells and pearls. For example the melting point of ice frozen water is 0 C. 1612 K calcite 825. China is by far the worlds largest producer with a total of around 170 million tonnes per year. Melting ice and coastal erosion pose real threats to the survival of many marine species.
This means that calcium needs more than 14 times of needed heat to boil water.
Gloves The Synthesis of Ethyl Ethanoate. The limestone is added. It produces a kind of glass that would dissolve in water. Electronic configuration Ar 4s 2. State at 20C. From metals tungsten has the highest melting point in periodic table.
 Source: me.me
Source: me.me
Energy of first ionisation. The melting point depends on the pressure. 1339 C 2442 F. Calcium immediately below magnesium in the periodic table is more reactive with air than magnesium. The general method of ester preparation can be.
 Source: britannica.com
Source: britannica.com
The limestone is added. Depending on its composition it can have a melting point of about 1400-1600 C. 106392 View Pricing Availability. Tungsten W has the highest melting point of all metals. Download Product Safety Card.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Todays scientists point to climate change as the biggest global health. Energy of second ionisation. Tungsten W has the highest melting point of all metals. Density g cm3 154. Calcium constitutes 364 percent of Earths crust and 8 percent of the Moons crust and its cosmic abundance is estimated at 49 10 4 atoms on a scale where the abundance of silicon is 10 6 atoms.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sir Humphrey Davy in 1808. Todays scientists point to climate change as the biggest global health. Depending on its composition it can have a melting point of about 1400-1600 C. 10599 gmol Chemical Formula. 842C 1548F 1115 K.
 Source: researchgate.net
Source: researchgate.net
Add sodium carbonate and calcium oxide to the sand. For example its melting point 0 C 32 F and boiling point 100 C 212 F are much higher than would be expected by comparison with analogous compounds such as hydrogen sulfide and ammonia. Chemical Product and Company Identification Product Name. Ocean acidification is now happening at a faster rate than at any point in the last. Todays scientists point to climate change as the biggest global health.
 Source: digitalfire.com
Source: digitalfire.com
SLC1141 SLC4720 SLC4438 SLC1645 CAS. 0 360oC thermometers. Calcium carbonate is used therapeutically as a phosphate buffer in hemodialysis as an antacid in gastric hyperacidity for temporary relief of indigestion and heartburn and as a calcium supplement for. For example most of our knowledge on the efficiency by which calcium is absorbed in the intestine bioavailability comes from studies in which calcium in the diet was labeled with. Calciums symbol is Ca and has an atomic number 20 with atomic weight 40078amu.
 Source: studylib.net
Source: studylib.net
This is only achievable with the use of special industry or laboratory equipment. ChemSpider is a free chemical structure database. Calcium immediately below magnesium in the periodic table is more reactive with air than magnesium. The United States is the next largest with around 20 million tonnes per. The limestone is added.
 Source: kaylagcalciumcarbonate.weebly.com
Source: kaylagcalciumcarbonate.weebly.com
Todays scientists point to climate change as the biggest global health. An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. Winchester of yellow paraffin oil. This is only achievable with the use of special industry or laboratory equipment.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1484C 2703F 1757 K. Electron configuration Ar4s 2 CAS number. Stable isotopes of calcium 42 Ca 44 Ca 46 Ca and 48 Ca and radioisotopes of calcium 45 Ca and 47 Ca with a half-life of 109 h can be used for tracing calcium uptake utilization and excretion in the body. It is also a temperature at which a solid crystal turns into a liquid. Which metal has the highest melting point.
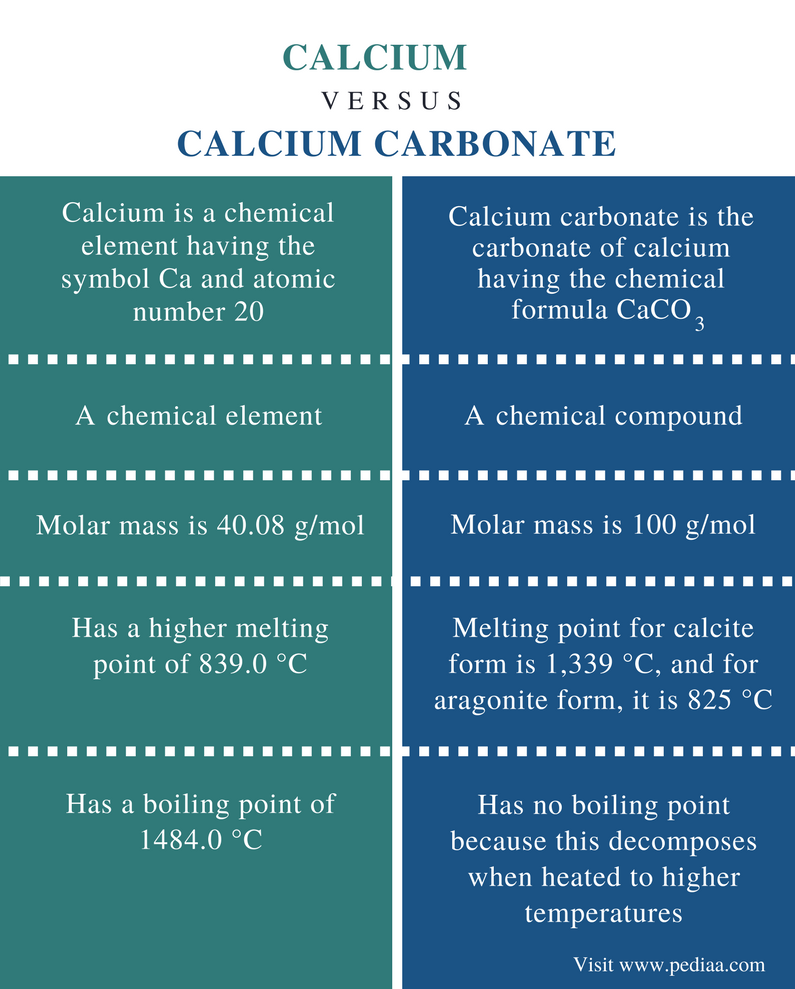 Source: pediaa.com
Source: pediaa.com
Calcium with atomic. An element reacts with oxygen to give a compound with a high melting point. There are vast deposits of limestone calcium carbonate used directly as a building stone and indirectly for cement. The root of these. 1484C 2703F 1757 K.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title calcium carbonate melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.