Cacl2 boiling point
Home » datasheet » Cacl2 boiling pointCacl2 boiling point
Cacl2 Boiling Point. This agent may also inhibit acid production by commensal oral bacteria. The boiling point of a solution is. The actual mass of the particle is irrelevant since a small molecule will exert the same effect as a large molecule. Which of the following statements about colligative properties is FALSE.
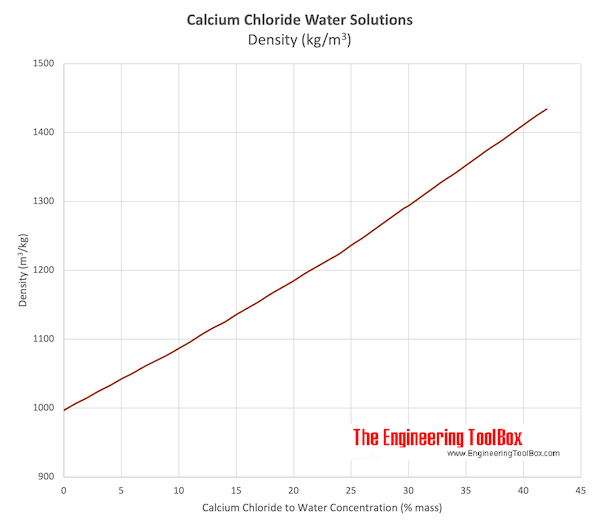 Calcium Chloride Water Solution From engineeringtoolbox.com
Calcium Chloride Water Solution From engineeringtoolbox.com
Water has is H2O. Boilingcondensation point Meltingfreezing point Molecular weight Critical temperature Molecular formula Ca-Cl2. Cover with drop of fresh stain. Which substance has the highest boiling point. The melting point of sodium hydroxide is 318C 604F without breakdown and its boiling point is 2530C 1388C. Crystalline and colourless it is solid.
The CaCl2 solution has the higher boiling point and the lower freezing point.
Atomic Weight of Calcium 4008 Atomic Weight of Chlorine 3545 CaCl2 is is 1101. The solvent is highly dispersible in water but less so in cold solvents such as methanol and ethanol. The CaCl2 solution has the higher boiling point and the lower freezing point. Senyawa ini merupakan ester dari etanol dan asam asetat. What does the chemical formula cacl2 show about the compound it represents. The change in temperature is proportional to the molality.
 Source: youtube.com
Source: youtube.com
Senyawa ini merupakan ester dari etanol dan asam asetat. Add additional drop of stain if needed. Calcium chloride is an inorganic compound a salt with the chemical formula CaCl 2It is a white colored crystalline solid at room temperature and it is highly soluble in water. 30000ppm is 3000 thousand parts per million Same as 30 parts per thousand. The CaCl2 solution has the higher boiling point and the lower freezing point.
 Source: hydro-land.com
Source: hydro-land.com
CO2 F2 FeCl2 PCl3. Look for cells which are well-separated not elongated and have conspicuous nuclei. Atomic Weight of Calcium 4008 Atomic Weight of Chlorine 3545 CaCl2 is is 1101. Both solutions have the same boiling point and the same freezing point. Apply cover slip and observe under relatively low power.

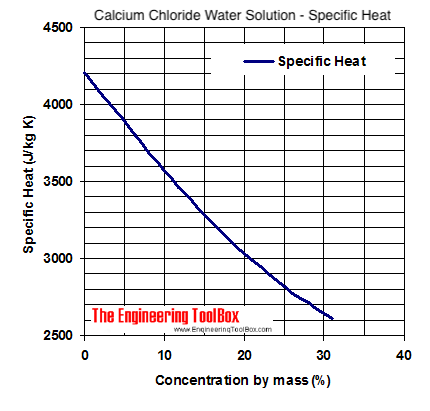
Calcium Chloride to Water Concentration by mass weight Specific Gravity at 60 o F 156 o C Freezing Point Boiling Point o Fo Co Fo C40. Hazardous in case of skin contact irritant of. Solvents other than NaOH are insoluble in NaOH. Is the intermolecular force present in HCl. Apply cover slip and observe under relatively low power.
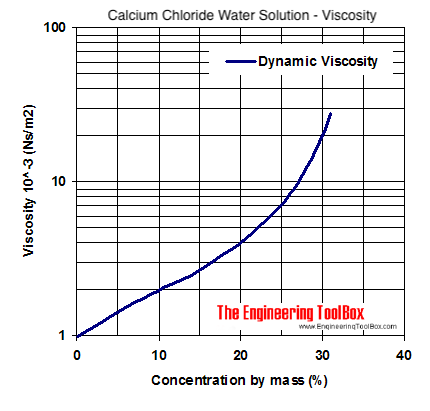 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The boiling point of methanol is 65 degrees Celsius and the boiling point of ethanol is 78 degrees Celsius. In these regions look for dividing. Hydrogen bonding is responsible for. The person who answered this question first was wrong. Which of the following statements is true.
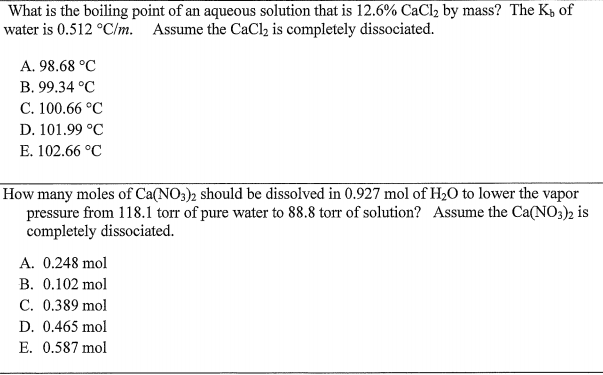 Source: chegg.com
Source: chegg.com
H20 NH4NO3 — NH4 NO3- Thank you for your time. CaCl2 2H2O What was the molarity of the HCl solution. Lastly dispersion forces act on any two adjacent molecules in a liquid and H2SO4 is a Dec 10 2014 Justin Dupuis Alex December 2014 SCH4U Example. Has one calcium atom two chlorine. Which are the strongest intermolecular forces.
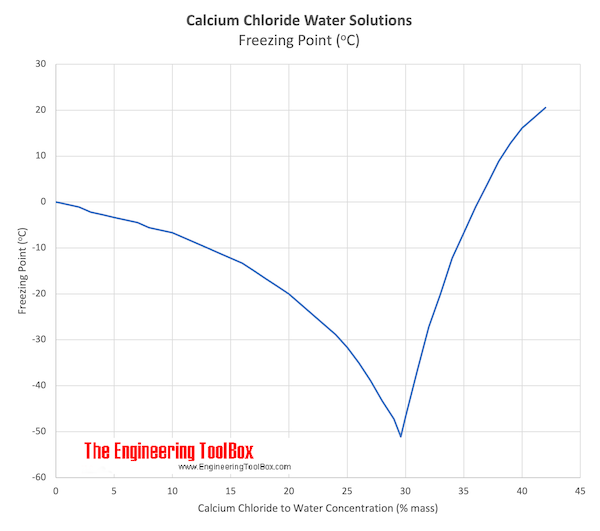 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Which of the following would have the higher boiling point. Etil asetat adalah senyawa organik dengan rumus empiris CH3COOC2H5. CH3CN C3H8 They have the same boiling point. A large amount of heat is released during the dissolution of solid sodium. Is cacl2 soluble in water email protected.
 Source: clutchprep.com
Source: clutchprep.com
Lastly dispersion forces act on any two adjacent molecules in a liquid and H2SO4 is a Dec 10 2014 Justin Dupuis Alex December 2014 SCH4U Example. 2 See answers Advertisement Advertisement OlaMacgregor OlaMacgregor Explanation. HCl HF HBr they have the same boiling point. The CaCl2 solution has the higher boiling point and the lower freezing point. Specific Volume ft 3lb03981 Gas Density lbft 3 2512 25C 77 to F Powered by IHS Date of issueDate of revision5182015Date of previous issue5182015.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The structure of calcium chloride molecules is illustrated blow. Calcium chloride is an inorganic compound a salt with the chemical formula CaCl 2It is a white colored crystalline solid at room temperature and it is highly soluble in water. Ethanol C2H5 OH will have a greater viscosity than ethylene. Solvents other than NaOH are insoluble in NaOH. Hazardous in case of skin contact irritant of.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Catharanthus roseus L G. An inexperienced student adds a solution of catalytic h2so4 and water to the alkene shown below expecting to produce 1-cyclobutylethan-1-ol. Specific GravityDensity21500 gcm3 Molecular FormulaCaCl2 Molecular Weight11099 Section 10 - Stability and Reactivity Chemical Stability. Heat for 60 seconds without boiling or drying. Osmolality is the number of Osmols of solute particles per kilogram of pure solvent.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
CO2 F2 FeCl2 PCl3. It can be noted that the calcium cation holds a charge of magnitude 2 and each chloride anion holds a charge of. Crystalline and colourless it is solid. It can be created by neutralising hydrochloric acid with calcium hydroxide. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title cacl2 boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.