C2h4 boiling point
Home » datasheet » C2h4 boiling pointC2h4 boiling point
C2h4 Boiling Point. This is because they have no interaction or polarity. Salt never melted and water has a lower boiling point so salt has stronger intramolecular forces. Determine 𝐻𝑓𝑜 for C2H4g given that the standard enthalpies of formation for CO2g and H2Ol are -3935 kJmol and -2858 kJmol respectively. E independent variables in Part I the ones intentionally.
 Ethylene Wikipedia From en.wikipedia.org
Ethylene Wikipedia From en.wikipedia.org
005 m C12H22O11 sucrose Assuming the volume outside the semipermeable membrane is large as the illustration shows what would you expect for the solution volume inside the balloon once the system has come to equilibrium through osmosis. 012 m C2H4OH2 ethylene glycol A. Throughout the reflection make sure you have a copy of the Student Guide and your data tables. What is the lewis structure for CH3COOH. Calculate the number of grams of an impurity of M100 gmole which be required to raise the boiling point of 50 grams of chloroform by this amount A. 005 m CaCl2 C.
005 m C12H22O11 sucrose Assuming the volume outside the semipermeable membrane is large as the illustration shows what would you expect for the solution volume inside the balloon once the system has come to equilibrium through osmosis.
What is the lewis structure for CH3COOH. Salt is a solid and water is a liquid so water has stronger intramolecular forces. The boiling point of chloroform can be measured with a particular apparatus with an accuracy of 001C. Calculate the number of grams of an impurity of M100 gmole which be required to raise the boiling point of 50 grams of chloroform by this amount A. Try to arrange the atoms to yield the most typical number of bonds for each atom. What is the lewis structure for CH3COOH.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Draw the best Lewis Dot Structure for each of the following species. 005 m CaCl2 C. Which solution will have the lowest boiling point. Nonpolar covalent bonds have a lower melting point and boiling point than nonpolar covalent bonds. The standard enthalpy for the combustion of one mole of ethane gas C2H4g is -14111 kJmol at 298K.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
005 m CaCl2 C. This is because they have no interaction or polarity. Differences in boiling and melting point. The first step in this calculation involves dividing the. Try to arrange the atoms to yield the most typical number of bonds for each atom.
 Source: thermopedia.com
Source: thermopedia.com
Differences in boiling and melting point. What is the lewis structure for CH3COOH. The boiling point of chloroform can be measured with a particular apparatus with an accuracy of 001C. 005 m C12H22O11 sucrose Assuming the volume outside the semipermeable membrane is large as the illustration shows what would you expect for the solution volume inside the balloon once the system has come to equilibrium through osmosis. This is because they have no interaction or polarity.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
005 m C12H22O11 sucrose Assuming the volume outside the semipermeable membrane is large as the illustration shows what would you expect for the solution volume inside the balloon once the system has come to equilibrium through osmosis. The first step in this calculation involves dividing the. 012 m C2H4OH2 ethylene glycol A. What is the lewis structure for CH3COOH. Use the drop-down menus to complete the statements.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The melting and boiling points of polar covalent bonds are higher than those of nonpolar covalent bonds. The first step in this calculation involves dividing the. Draw the best Lewis Dot Structure for each of the following species. The boiling point of chloroform can be measured with a particular apparatus with an accuracy of 001C. Use the drop-down menus to complete the statements.
 Source: chegg.com
Source: chegg.com
This is because they have no interaction or polarity. The physical properties of the molecule like boiling point surface tension etc. E independent variables in Part I the ones intentionally. Due to the unrestricted mobility of ions molecules with polar. Draw the best Lewis Dot Structure for each of the following species.
 Source: sites.google.com
Source: sites.google.com
The first step in this calculation involves dividing the. Nonpolar covalent bonds have a lower melting point and boiling point than nonpolar covalent bonds. Determine 𝐻𝑓𝑜 for C2H4g given that the standard enthalpies of formation for CO2g and H2Ol are -3935 kJmol and -2858 kJmol respectively. What is the lewis structure for CH3COOH. 005 m CaCl2 C.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
11 Adapted Stein Brown method Melting Pt deg C. The physical properties of the molecule like boiling point surface tension etc. 005 m NaCl D. The melting and boiling points of polar covalent bonds are higher than those of nonpolar covalent bonds. Salt never melted and water has a lower boiling point so salt has stronger intramolecular forces.
 Source: en.wikipedia.org
Source: en.wikipedia.org
012 m C2H4OH2 ethylene glycol A. 005 m NaCl D. Calculate the number of grams of an impurity of M100 gmole which be required to raise the boiling point of 50 grams of chloroform by this amount A. The physical properties of the molecule like boiling point surface tension etc. Differences in boiling and melting point.
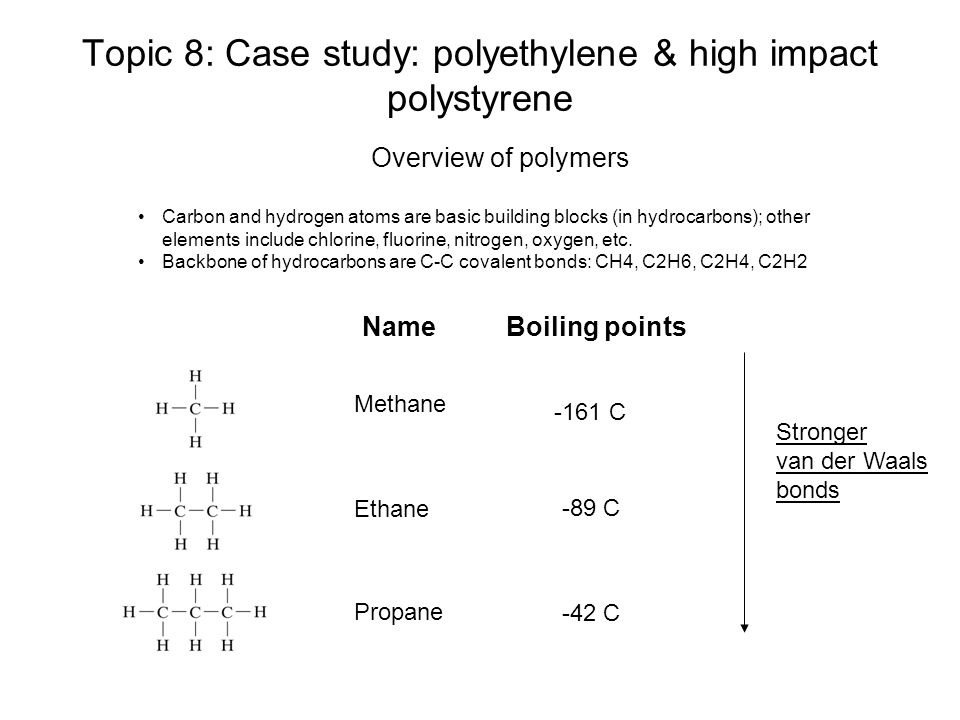 Source: slideplayer.com
Source: slideplayer.com
Try to arrange the atoms to yield the most typical number of bonds for each atom. Draw the best Lewis Dot Structure for each of the following species. Nonpolar covalent bonds have a lower melting point and boiling point than nonpolar covalent bonds. 005 m C12H22O11 sucrose Assuming the volume outside the semipermeable membrane is large as the illustration shows what would you expect for the solution volume inside the balloon once the system has come to equilibrium through osmosis. Calculate the number of grams of an impurity of M100 gmole which be required to raise the boiling point of 50 grams of chloroform by this amount A.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title c2h4 boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.