Butyl methyl ether boiling point
Home » datasheet » Butyl methyl ether boiling pointButyl methyl ether boiling point
Butyl Methyl Ether Boiling Point. 51 C 124 F. Butyl glycol also known as BG 2-butoxyethanol glycol monobutyl ether and ethylene glycol monobutyl ether butyl cellosolve butoxyethanol is a clear colourless oily liquid with a unique sweet yet mild odour and has the formula C 6 H 14 O 2. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. It is a precursor to many other compounds of commercial interest.
 Tert Butyl Methyl Ether Anhydrous 99 8 1634 04 4 From sigmaaldrich.com
Tert Butyl Methyl Ether Anhydrous 99 8 1634 04 4 From sigmaaldrich.com
A gas chromatographic method for the analysis of ethyl ether consists of a stainless steel column 12 m x 6 mm OD packed with Porapak Q 5080 mesh with hydrogen-air flame ionization detection and nitrogen as the carrier gas at a flow rate of 30 mlmin is a NIOSH approved method. A sample injection volume of 5 ul is suggested the column temperature is 175 C the. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents. Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. Still the attractive forces in butanol pale in. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases.
All boiling points below are normalatmospheric boiling points.
All boiling points below are normalatmospheric boiling points. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents. Still the attractive forces in butanol pale in. It is a precursor to many other compounds of commercial interest. The simplest example of an ester it is a colorless liquid with an ethereal odour high vapor pressure and low surface tension. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
P210 P233 P240 P241 P242 P243 P280 P303P361P353 P370P378 P403P235 P501. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases. The simplest example of an ester it is a colorless liquid with an ethereal odour high vapor pressure and low surface tension. It is a precursor to many other compounds of commercial interest. NFPA 704 fire diamond 2.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases. It has been produced industrially for over half a. NFPA 704 fire diamond 2. Methyl formate also called methyl methanoate is the methyl ester of formic acid. All boiling points below are normalatmospheric boiling points.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C. Methyl formate also called methyl methanoate is the methyl ester of formic acid. A sample injection volume of 5 ul is suggested the column temperature is 175 C the. It is a precursor to many other compounds of commercial interest.
 Source: scbt.com
Source: scbt.com
324 K Solubility in water. P210 P233 P240 P241 P242 P243 P280 P303P361P353 P370P378 P403P235 P501. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. It has been produced industrially for over half a.
 Source: guidechem.com
Source: guidechem.com
It has been produced industrially for over half a. Butyl glycol also known as BG 2-butoxyethanol glycol monobutyl ether and ethylene glycol monobutyl ether butyl cellosolve butoxyethanol is a clear colourless oily liquid with a unique sweet yet mild odour and has the formula C 6 H 14 O 2. Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents. Methyl formate also called methyl methanoate is the methyl ester of formic acid.
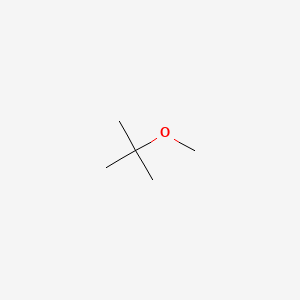
A gas chromatographic method for the analysis of ethyl ether consists of a stainless steel column 12 m x 6 mm OD packed with Porapak Q 5080 mesh with hydrogen-air flame ionization detection and nitrogen as the carrier gas at a flow rate of 30 mlmin is a NIOSH approved method. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. It is a precursor to many other compounds of commercial interest. GHS precautionary statements. 349 kPa 20 C Hazards GHS pictograms.

324 K Solubility in water. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Methyl formate also called methyl methanoate is the methyl ester of formic acid. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases.
 Source: tcichemicals.com
Source: tcichemicals.com
Butyl glycol also known as BG 2-butoxyethanol glycol monobutyl ether and ethylene glycol monobutyl ether butyl cellosolve butoxyethanol is a clear colourless oily liquid with a unique sweet yet mild odour and has the formula C 6 H 14 O 2. P210 P233 P240 P241 P242 P243 P280 P303P361P353 P370P378 P403P235 P501. NFPA 704 fire diamond 2. Butyl glycol also known as BG 2-butoxyethanol glycol monobutyl ether and ethylene glycol monobutyl ether butyl cellosolve butoxyethanol is a clear colourless oily liquid with a unique sweet yet mild odour and has the formula C 6 H 14 O 2. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents.

Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. It is a precursor to many other compounds of commercial interest. NFPA 704 fire diamond 2. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases. The simplest example of an ester it is a colorless liquid with an ethereal odour high vapor pressure and low surface tension.
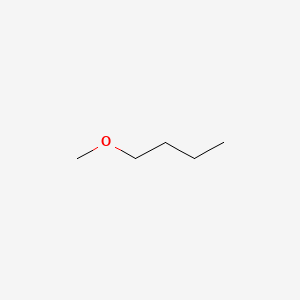
51 C 124 F. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. NFPA 704 fire diamond 2. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C. A gas chromatographic method for the analysis of ethyl ether consists of a stainless steel column 12 m x 6 mm OD packed with Porapak Q 5080 mesh with hydrogen-air flame ionization detection and nitrogen as the carrier gas at a flow rate of 30 mlmin is a NIOSH approved method.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title butyl methyl ether boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.