Butanol boiling point
Home » datasheet » Butanol boiling pointButanol boiling point
Butanol Boiling Point. Product Identification Synonyms. The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. This is significant in cooling the car since as soon as the water in the car radiator boilsit does not work anymore as a coolant. Hessel and Geiseler 1965.
 Amf From iea-amf.org
Amf From iea-amf.org
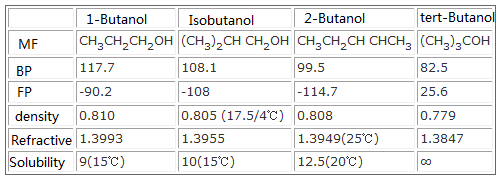
Hessel and Geiseler 1965. Fuels - Densities and Specific Volumes - Densities and specific volumes fuels like anthracite butane gasoil diesel coke oil wood and more. N-butanol CH3CH2CH2CH2OH 8 n-pentanol CH3CH2CH2CH2CH2OH 2 n-hexanol CH3CH2CH2CH2CH2CH2OH 05 n-pentanol CH3CH2CH2CH2CH2CH2CH2OH 01 Compounds with six or more carbons for each polar group will not be very soluble in polar solvents but will be soluble in non. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. Alcohol - ethyl grain ethanol C. Boiling Point and Dipole-Dipole Interactions.
Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly.
A clear colorless solution with a sweet musty odor. A clear high-boiling point and low volatiity solvent with a characteristic color. It is one of several isomers of amyl alcohol pentanol. Isoamyl alcohol is an ingredient in the production of. This is significant in cooling the car since as soon as the water in the car radiator boilsit does not work anymore as a coolant. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water.
The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. This is significant in cooling the car since as soon as the water in the car radiator boilsit does not work anymore as a coolant. Subsequently the structure of the Company has changed into an assisted sector Company with ASL. CH3CH22CH2OH COMPANY IDENTIFICATION Supplier. A colorless neutral liquid of medium volatiity with a characteristic banana like odor.
 Source: en.wikipedia.org
Source: en.wikipedia.org
N-butanol CH3CH2CH2CH2OH 8 n-pentanol CH3CH2CH2CH2CH2OH 2 n-hexanol CH3CH2CH2CH2CH2CH2OH 05 n-pentanol CH3CH2CH2CH2CH2CH2CH2OH 01 Compounds with six or more carbons for each polar group will not be very soluble in polar solvents but will be soluble in non. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. Quadratic Formula Circumference Formula Compound Interest Formula Midpoint Formula Arc Length Formula Area of a Triangle Formula Exponential Growth Formula Percent Change Formula Point-slope formula Simple Interest Formula. A colorless neutral liquid of medium volatiity with a characteristic banana like odor.
 Source: chemicalbook.com
Source: chemicalbook.com
Read More Normal Butanol. Temperature K A B C Reference Comment. 7412 Chemical Formula. Acetic acid anhydride CH 3 COO 2 O. 71 -36 -3 Molecular Weight.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Volunteers exposed to n-butanol for 2 hr at air concn of 100 and 200 ppm developed blood concn that never exceeded 10 mgL whether at rest or during excercise. It is one of several isomers of amyl alcohol pentanol. But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. Acetic acid anhydride CH 3 COO 2 O. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure.
 Source: iea-amf.org
Source: iea-amf.org
The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. Of these two the boiling point is considered the most representative measure of general intermolecular attractions. A colorless neutral liquid of medium volatiity with a characteristic banana like odor. Alcohol - ethyl grain ethanol C. The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Of these two the boiling point is considered the most representative measure of general intermolecular attractions. Fuels - Boiling Points - Fuels and their boiling points. Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. Immediately flush eyes. Isoamyl alcohol is an ingredient in the production of.
 Source: en.wikipedia.org
Source: en.wikipedia.org
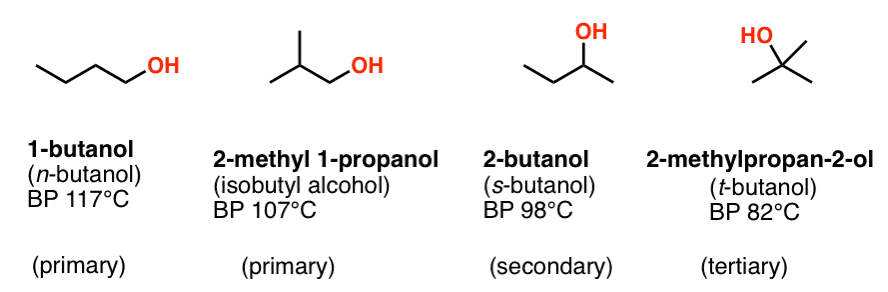
The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Temperature K A B C Reference Comment. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol.
 Source: iea-amf.org
Source: iea-amf.org
Immediately flush eyes. 71 -36 -3 Molecular Weight. CH3CH22CH2OH COMPANY IDENTIFICATION Supplier. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol. 23 K to 353.
 Source: thermopedia.com
Source: thermopedia.com
23 K to 353. A clear high-boiling point and low volatiity solvent with a characteristic color. We mentioned in the previous post that stronger intermolecular interactions increase the boiling and melting points but how exactly they affect the physical properties might be your next question. Fuels - Combustion Air and Flue Gases - Combustion air and flue gas for common fuels - coke oil wood natural gas and more. Of these two the boiling point is considered the most representative measure of general intermolecular attractions.

The distance between molecules in a crystal lattice is small and regular with intermolecular forces serving to constrain. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. It is one of several isomers of amyl alcohol pentanol. The freezing point depression aspectcan also be applied in daily life for example in raising the boiling point of the water that cools cars.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title butanol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.