Butane boiling point
Home » datasheet » Butane boiling pointButane boiling point
Butane Boiling Point. It is common to distribute a mixture of propane C 3 H 8 and butane C 4 H 10 for combustion purposesPropane is more suited to colder environments since it evaporates at -44 o F -42 o C at atmospheric pressure. Highly branched vs. Smaller volumes 12 mL. If this all seems rather ambiguous contradictory and imprecise well you have a point.
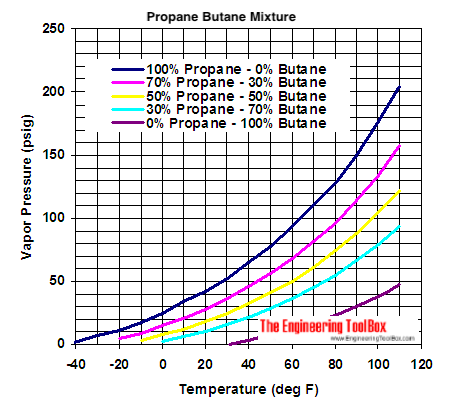 Propane Butane Mixture Evaporation Pressure From engineeringtoolbox.com
Propane Butane Mixture Evaporation Pressure From engineeringtoolbox.com
Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. N-butane like Puretane butane is highly refined and is the kind of butane we usually think of when we hear the word. It is common to distribute a mixture of propane C 3 H 8 and butane C 4 H 10 for combustion purposesPropane is more suited to colder environments since it evaporates at -44 o F -42 o C at atmospheric pressure. NIOSH REL TWA 800 ppm 1900 mgm³ OSHA PEL none See Appendix G. If this all seems rather ambiguous contradictory and imprecise well you have a point. Melting Point and Boiling point- Melting point is a characteristic property of solid crystalline substances.
Conversely butane is typically used indoors due to its higher boiling point.
For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. Branched more sphere-like - lower surface area lower boiling point. 1 ppm 238 mgm³ IDLH. Liquefied petroleum gas LPG or LP gas is also referred to by its constituent names propane or butane. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F. Natural gas is only 387MJm³.
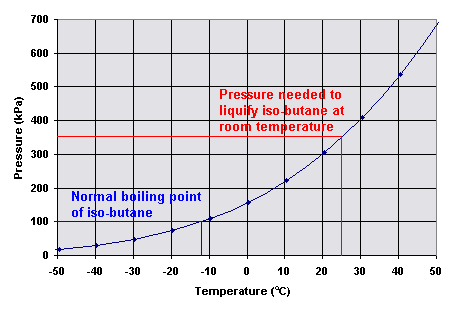 Source: digipac.ca
Source: digipac.ca
So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F. Butane is perfect for any of your portable gas heaters and single-burner cooking appliances. 1011 115 1075 115. It is common to distribute a mixture of propane C 3 H 8 and butane C 4 H 10 for combustion purposesPropane is more suited to colder environments since it evaporates at -44 o F -42 o C at atmospheric pressure. Also see specific listing for Isobutane CAS No.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Compare the different butane alcohol derivatives shown below. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. Compare its boiling point with that of n-butanol. Also see specific listing for Isobutane CAS No. Normal-Butane Butyl hydride Diethyl Methylethylmethane Note.
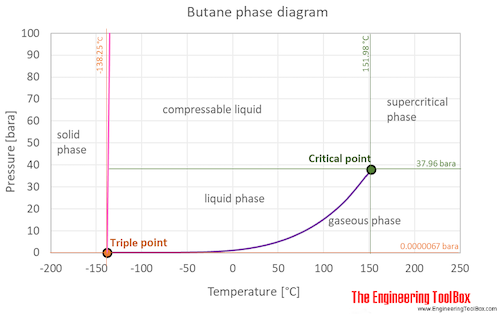 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. Smaller volumes 12 mL. N-butane like Puretane butane is highly refined and is the kind of butane we usually think of when we hear the word. Conversely butane is typically used indoors due to its higher boiling point. As a final example I give you 2233-tetramethylbutane.
 Source: thermopedia.com
Source: thermopedia.com
The boiling point of n-butanol is 117 o C. An isomer of isooctane gasoline with melting point 95 C. Liquefied petroleum gas LPG or LP gas is also referred to by its constituent names propane or butane. As a final example I give you 2233-tetramethylbutane. Propane has a lower boiling point at -42 vs -04C for butane.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Butane or C4H10 is a derivative of alkane natural gas that can be found as two separate structural isomers n-butane or isobutane or a combination of the two. The boiling point temperature will be lower if the atmospheric pressure is decreased. Natural gas is only 387MJm³. This follows ASTM method D 1310. Branched more sphere-like - lower surface area lower boiling point.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Particularly for operating tools ovens or forklift trucks for example. As a final example I give you 2233-tetramethylbutane. Conversely butane is typically used indoors due to its higher boiling point. An isomer of isooctane gasoline with melting point 95 C. This follows ASTM method D 1310.
 Source: thermopedia.com
Source: thermopedia.com
For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. Further proof as if more was needed that. Butane or C4H10 is a derivative of alkane natural gas that can be found as two separate structural isomers n-butane or isobutane or a combination of the two. NIOSH REL TWA 800 ppm 1900 mgm³ OSHA PEL none See Appendix G. Liquefied petroleum gas LPG or LP gas is also referred to by its constituent names propane or butane.
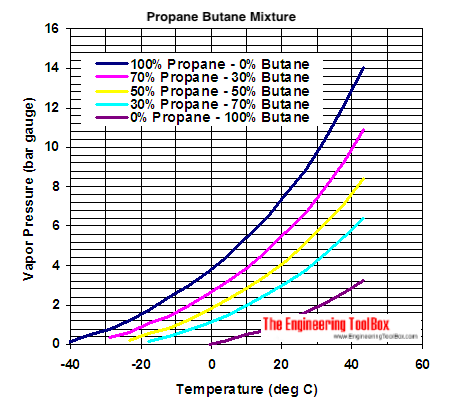 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen. 1 ppm 238 mgm³ IDLH. It is common to distribute a mixture of propane C 3 H 8 and butane C 4 H 10 for combustion purposesPropane is more suited to colder environments since it evaporates at -44 o F -42 o C at atmospheric pressure. Liquefied petroleum gas LPG or LP gas is also referred to by its constituent names propane or butane. Its not a straightforward topic.
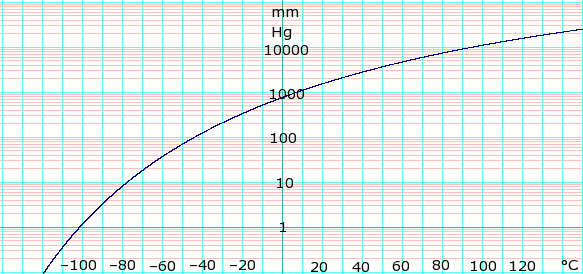 Source: en.wikipedia.org
Source: en.wikipedia.org
The flash point of an oil product can be determined by several methods depending on the oil product and the quantity available. Its also well suited for providing. N-butane like Puretane butane is highly refined and is the kind of butane we usually think of when we hear the word. Normal-Butane Butyl hydride Diethyl Methylethylmethane Note. Its not a straightforward topic.
 Source: researchgate.net
Source: researchgate.net
The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen. Also see specific listing for Isobutane CAS No. As a final example I give you 2233-tetramethylbutane. Propane is the favoured choice for commercial use. Normal-Butane Butyl hydride Diethyl Methylethylmethane Note.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title butane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.