Butanal boiling point
Home » datasheet » Butanal boiling pointButanal boiling point
Butanal Boiling Point. Acetic acid anhydride CH 3 COO 2 O. The reaction acarbocation formation b free-radical mechanism c carbanion. 9 09 Turn over IBMJun1874042 Do not write. It occurs as a volatile constituent in olives.

Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. If you are not found for Sig Sauer Ase Black simply check out our information below. Direction for questions from 8 to 14. Isovaleraldehyde organic compound also known as 3. Find books Drawing Electron-Dot and Line-Bond Structures Draw both electron-dot and line-bond structures for chloromethane CH3Cl. It has a role as a flavouring agent a plant metabolite a volatile oil component and a Saccharomyces cerevisiae metabolite.
Less dense than water and insoluble in water.
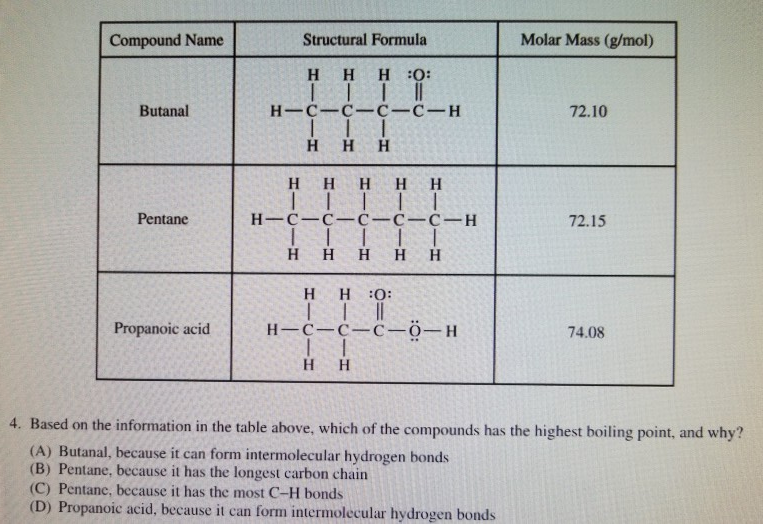
Store in cool dry place in tightly sealed containers protected from heat and. The carbon atoms in saturated hydrocarbons _____. Boiling point Solubility in water Pentane 72 gmol 35C Insoluble Diethyl ether 74 gmol 35C Insoluble Butanal 72 gmol 76C 71 g 100 mL H2O 1-Butanol 74 gmol 118C 91 g 100 mL H2O Propanoic acid 74 gmol 141C Infinite Boiling Point. 3-methylbutanal is a methylbutanal that is butanal substituted by a methyl group at position 3. The a structural b condensed structural and c molecular formula representations of butanal. Soluble in alcohol and ether slightly soluble in water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Boiling point increases with increase in molecular mass and decreases with increase in branching. Soluble in alcohol and ether slightly soluble in water. See also Autoignition temperature and flash point of different hydrocarbons. Have only single bonds. The bond that undergoes heterolytic cleavage most readily is a C-C b C-O c C-H d O-H Question 5.

C Butane is non-polar and cannot form hydrogen bonds. It has a role as a biomarker an Escherichia coli metabolite and a mouse metabolite. Some uses of aldehydes include. Boiling point Solubility in water Pentane 72 gmol 35C Insoluble Diethyl ether 74 gmol 35C Insoluble Butanal 72 gmol 76C 71 g 100 mL H2O 1-Butanol 74 gmol 118C 91 g 100 mL H2O Propanoic acid 74 gmol 141C Infinite Boiling Point. 1 of an alkane in terms of.
 Source: doubtnut.com
Source: doubtnut.com
TCC 9889 C. CH3CH2CHO Boiling point is the 3. Fuel Flash Point o F Acetaldehyde-36. 2400 months or longer if stored properly. Carboxylic acid Alcohols AldehydesKetones Ethers Alkanes Water Solubility.
 Source: study.com
Source: study.com
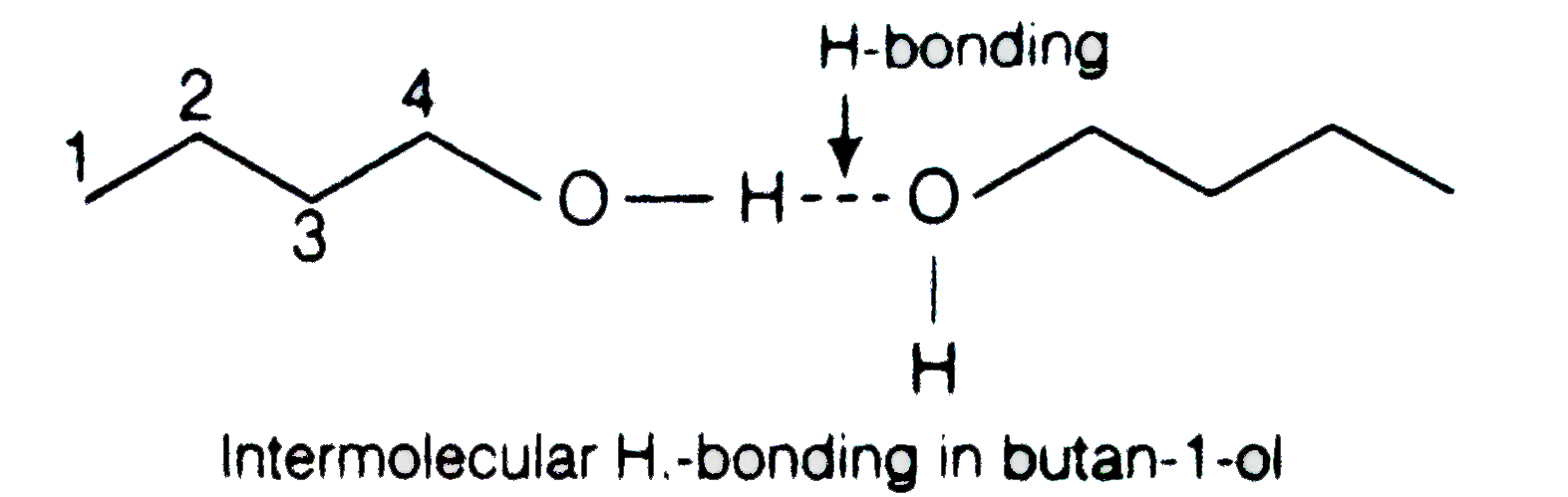
Low boiling point high flammability high melting point. If you are not found for Sig Sauer Ase Black simply check out our information below. Butanal is a member of the class of butanals that consists of propane bearing a formyl substituent at the 1-position. Fuel Flash Point o F Acetaldehyde-36. Why is there a large difference in th boiling points of butanal and butan-1-ol.
 Source:
Source:
Boiling Point Water Solubility CH 3 2 CCH 2. The a structural b condensed structural and c molecular formula representations of butanal. Boiling point 757F Hawleys. Why is there a large difference in th boiling points of butanal and butan-1-ol. 3-methylbutanal is a methylbutanal that is butanal substituted by a methyl group at position 3.
 Source: doubtnut.com
Source: doubtnut.com
E 3-chloro-1-butanal f 3-penten-2-one g 2-methyl-3-hexen-1-al h 3-ethyl-3-methyl-1-pentanol i 24-dimethyl-24-hexandiol j tetrachloroethene Question 3. C Butane is non-polar and cannot form hydrogen bonds. Isovaleraldehyde is a colorless liquid with a weak suffocating odor. Explain why an organic liquid vaporises at a temperature below its boiling point in its steam distillation. Boiling point increases with increase in molecular mass and decreases with increase in branching.
 Source: chemistrysteps.com
Source: chemistrysteps.com
CH 3 CH 2 CH 2 CHCH 2. Butanal has no intermolecular hydrogen bonding but butan-1-ol has intermolecular hydrogen bonding. View solution I 1 2-Dihydroxybenzene II 1 3-Dihydroxybenzene III 1 4-Dihydroxybenzene IV Hydroxybenzene The increasing order of. Soluble in alcohol and ether slightly soluble in water. Boiling point of rainwater is less than that of sea water.
 Source: chegg.com
Source: chegg.com
The boiling point is the temperature at which the vapor pressure is equal to the external pressure. Carboxylic acid Alcohols AldehydesKetones Ethers Alkanes Water Solubility. Boiling point of rainwater is less than that of sea water. 1-propanol is polar. From Grams to Tons.
12600 to 12700 C. Functional groups may have strong hydrogen bonds. Contain at least one triple bond have only single bonds contain a benzene ring contain at least one double bond contain both a double and a triple bond. Boiling Point As the carbonyl group contains dipole-dipole forces they have a higher boiling point than their corresponding alkanes but a lower boiling point then their corresponding alcohols. If you are not found for Sig Sauer Ase Black simply check out our information below.

Compare boiling points energy required to overcome intermolecular force of aldehydesB and alkanesC. 2-methyl butanal b butan-2-aldehyde c 2-ethylpropanal d 3-methyl isobutraldehyde Question 4. It has a role as a biomarker an Escherichia coli metabolite and a mouse metabolite. Fuel Flash Point o F Acetaldehyde-36. We mentioned in the previous post that stronger intermolecular interactions increase the boiling and melting points but how exactly they affect the physical properties might be your next question.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title butanal boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.