Br2 melting and boiling point
Home » datasheet » Br2 melting and boiling pointBr2 melting and boiling point
Br2 Melting And Boiling Point. Q3 Rank from lowest to highest boiling point. Q2 To go from a liquid to a gas what must happen. We would like to show you a description here but the site wont allow us. Why is the boiling point of water so much higher than that of methane.
 Bromine Wikipedia From en.wikipedia.org
Bromine Wikipedia From en.wikipedia.org
HFHF 20 C and HClHCl -85 C b. Surface tension of water liquids and aqueous solutions table of values Dielectric constant of liquids gases and solids Table Dissociation constants of acids and bases. The Goldfinger Mechanism for allylic bromination which proposed that NBS serves to provide a low concentration of Br2. All our academic papers are written from scratch. This fact is used as a test for the detection of sulphur dioxide. It is completely soluble in water.
Q2 To go from a liquid to a gas what must happen.
For example if you consider Cl 2 and Br2 you might expect the two compounds to behave similarly because they are both halogens. Specific GravityDensity31200gcm3 Molecular FormulaBr2 Molecular Weight15981 Section 10 - Stability and Reactivity Chemical Stability. Q2 To go from a liquid to a gas what must happen. Fisher Scientific 1 Reagent Lane Fair Lawn NJ 07410 For. It is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. The normal boiling point of dichloromethane is 40.
 Source: youtube.com
Source: youtube.com
Choose the substance with the highest boiling point. Has weaker intermolecular forces and is a gas at 300 mmHg Part B. Q4 Give an explanation in terms of intermolecular forces for the following differences in boiling point. All our academic papers are written from scratch. Which of the following compounds will be most soluble in pentane C5H12.
 Source: youtube.com
Source: youtube.com
Q2 To go from a liquid to a gas what must happen. NH3 a I II and III b II IV and V c I III and IV d I IV and V e II and V 5. If anyone if interested I just. Double and single spacing. Has weaker intermolecular forces and is a gas at 300 mmHg Part B.
 Source: toppr.com
Source: toppr.com
There is a colour change from purple pink in dilute solution to colourless on the addition of the gas to a solution of potassium manganate VII permanganate 2MnO4- 5SO 2H 2 O 2Mn 2 5SO4. Boiling point of liquids table of values Boiling point of water depending on pressure. 59 deg C FreezingMelting PointNot available. What advantages do you get from our Achiever Papers services. Mass of one atom of an element relative to one twelfth of the mass of one atom.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Log P octanol-water 1940 none EST. It should be noted that addition of Br to alkenes does occur to some extent but the reaction is reversible and if Br2 and HBr concentrations are kept low any. For example if you consider Cl 2 and Br2 you might expect the two compounds to behave similarly because they are both halogens. The molecular mass of ethanol is 46069 gmol1. NH3 a I II and III b II IV and V c I III and IV d I IV and V e II and V 5.
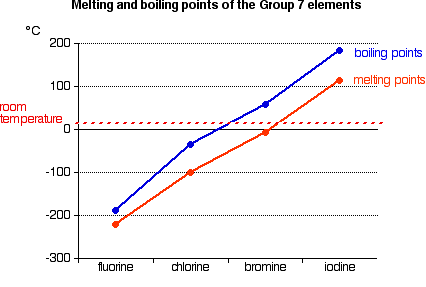 Source: chemguide.co.uk
Source: chemguide.co.uk
Relatively low melting point and boiling point. A CH4 b KI c CS2 d HF e I2 6. This fact is used as a test for the detection of sulphur dioxide. Q4 Give an explanation in terms of intermolecular forces for the following differences in boiling point. All our academic papers are written from scratch.
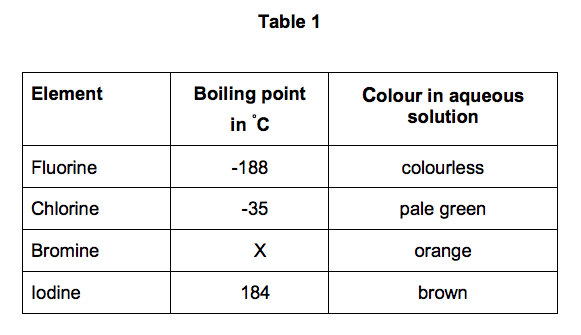 Source: elevise.co.uk
Source: elevise.co.uk
Br 2Br2 59 C and IClICl 97 C Author. What advantages do you get from our Achiever Papers services. Dont conduct electricity - have no mobile ions or electrons except for graphite Strength. Moist sulphur dioxide or sulphurous acid is a reducing agent. Mass of one atom of an element relative to one twelfth of the mass of one atom.
Source: chemspider.com
10 years in academic writing. It is a hard brittle bluish-white transition metal in the platinum group that is found as a trace element in alloys mostly in platinum ores. 85 10 average quality score from customers. NH3 a I II and III b II IV and V c I III and IV d I IV and V e II and V 5. Q3 Rank from lowest to highest boiling point.
 Source: clutchprep.com
Source: clutchprep.com
CH CH2CH2 CH2CH3 and C. It is a hard brittle bluish-white transition metal in the platinum group that is found as a trace element in alloys mostly in platinum ores. HFHF 20 C and HClHCl -85 C b. Which one of the following substances is expected to have the highest boiling point. Specific GravityDensity31200gcm3 Molecular FormulaBr2 Molecular Weight15981 Section 10 - Stability and Reactivity Chemical Stability.
 Source: youtube.com
Source: youtube.com
There is a colour change from purple pink in dilute solution to colourless on the addition of the gas to a solution of potassium manganate VII permanganate 2MnO4- 5SO 2H 2 O 2Mn 2 5SO4. In low concentration oxygen can act as a free-radical initiator forming Br radicals from Br2 but here Kharasch also observes that in high concentration oxygen can inhibit free-radical reactions. Q2 To go from a liquid to a gas what must happen. All our academic papers are written from scratch. Relatively low melting point and boiling point.
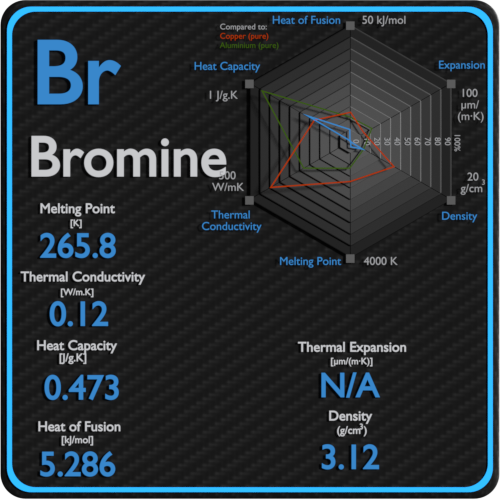 Source: material-properties.org
Source: material-properties.org
The density of ethanol is 789 gl that is around 20 less than that of water. Hi ionic or molecular. 宝塚の広告企画会社クルーズが年に4回発行している地域コミュニティ情報誌ComiPaコミパ 宝塚市のグルメやお稽古街の素敵な情報を発信 情報提供してくださる方バナー広告主様も募集中です. Which one of the following substances is expected to have the highest boiling point. Which one of the following substances is expected to have the lowest melting point.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title br2 melting and boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.