Boiling point trimethylamine
Home » datasheet » Boiling point trimethylamineBoiling point trimethylamine
Boiling Point Trimethylamine. The molecular weight and boiling point of compounds are directly related with the flavor development eg esters and aldehyde have higher molecular weight but lower boiling point but lactones have relatively high boiling point. Trimethylamine is a good nucleophile and this reaction is the basis of most of its applications. Use and alter these presentations freely or any power point template used in this presentations site for other teachers. Extremely Hazardous Substances EHS Chemical Profiles and Emergency First Aid Guides.
 Boiling Point And Melting Point In Organic Chemistry Chemistry Steps From chemistrysteps.com
Boiling Point And Melting Point In Organic Chemistry Chemistry Steps From chemistrysteps.com
Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Carboxylic acid Alcohols 12 Amines 3 AminesAlkanes. In a tertiary amine there arent any hydrogen atoms attached directly to the nitrogen. Oxidizer - oxygen or air. CH 3 CO 2 H. Alcohols boil considerably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen.
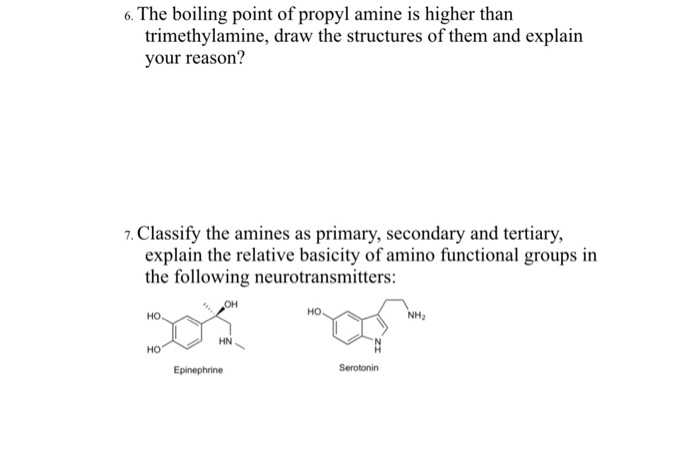
Fish proteins start to dry out and lose moisture at 140F60C and will be very dry at 160F71C.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. It is a tertiary amine and a member of methylamines. Trimethylamine is a good nucleophile and this reaction is the basis of most of its applications. As one might readily guess the inclusion of a heteroatom such as nitrogen in otherwise exclusively carbon and hydrogen molecules has quite an effect on the properties of amines as compared to alkanes. Thats why the boiling point is much lower.
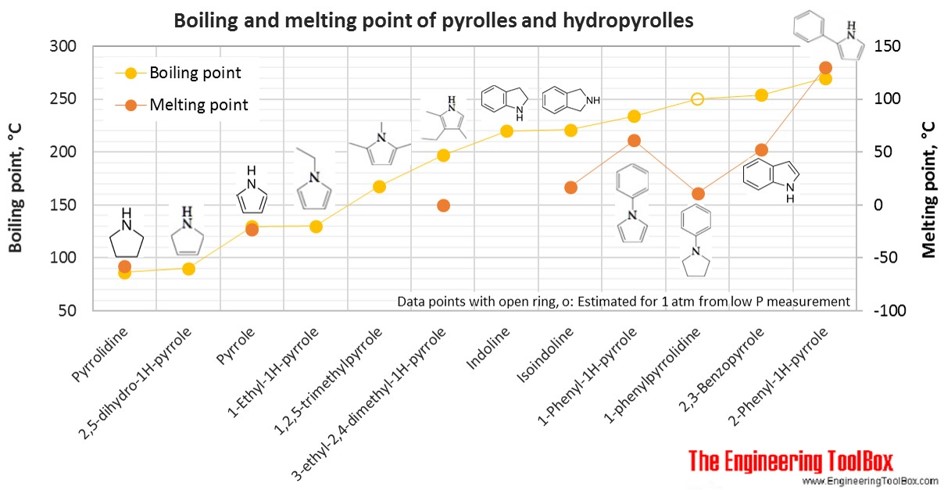 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Three basic requirements must be met for explosion to take place. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Chapter 6 Amines and Amides 19 Physical Properties of Amines. 1305 to 1334 F. The boiling point is specific for the given substanceFor example the boiling point of.
 Source: youtube.com
Source: youtube.com
For instance in the first example below the trisubstituted alkene is favoured over the mono-substituted alkene. Fish proteins start to dry out and lose moisture at 140F60C and will be very dry at 160F71C. In a few cartilaginous fish like shark and skate cooking to 140F60C is needed to soften their connective tissue. 1887 kPa at 20 C Henrys law. Trimethylamine CH 3 3 N.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Fats and gelatin in the muscles also melt at a lower point too. 1305 to 1334 F. The major product will be the more substituted alkene that is the alkene with the most carbons directly attached to the alkene. Fish proteins start to dry out and lose moisture at 140F60C and will be very dry at 160F71C. Carboxylic acid Alcohols 12 Amines 3 AminesAlkanes.
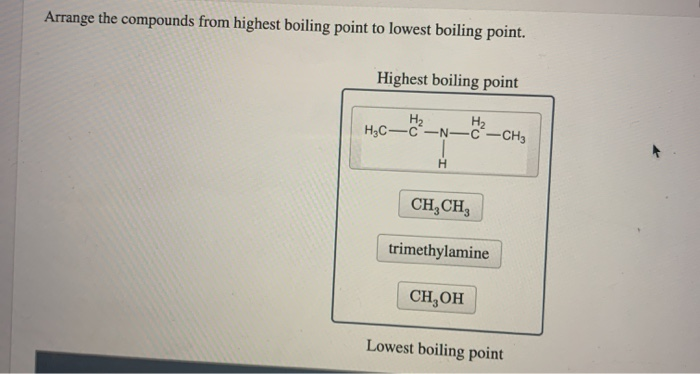 Source: chegg.com
Source: chegg.com
The boiling point is specific for the given substanceFor example the boiling point of. Chapter 6 Amines and Amides 19 Physical Properties of Amines. In a tertiary amine there arent any hydrogen atoms attached directly to the nitrogen. Trimethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an methyl group. Fish proteins start to dry out and lose moisture at 140F60C and will be very dry at 160F71C.
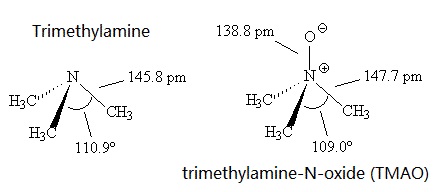 Source: chemicalbook.com
Source: chemicalbook.com
37 to 44 F. Boiling point Acetic acid 600 gmol 118C 1-propanol 601 gmol 97C propyl amine 591 gmol 48C ethylmethylamine 591 gmol 36C trimethylamine 591 gmol 29C butane 581 gmol -05C Boiling Point. 3279 to 3295 K Solubility in water. H 2 NCH 2 CH 2 NH 2. Fish proteins start to dry out and lose moisture at 140F60C and will be very dry at 160F71C.

Three basic requirements must be met for explosion to take place. Methylamine and trimethylamine while liquids are easily vaporized. Some fish including tuna and swordfish are. 242975 kPa Henrys law constant k H 150 μmol Pa 1 kg 1. Conventional elimination reactions that occur via the E2 mechanism follow Zaitsevs rule.
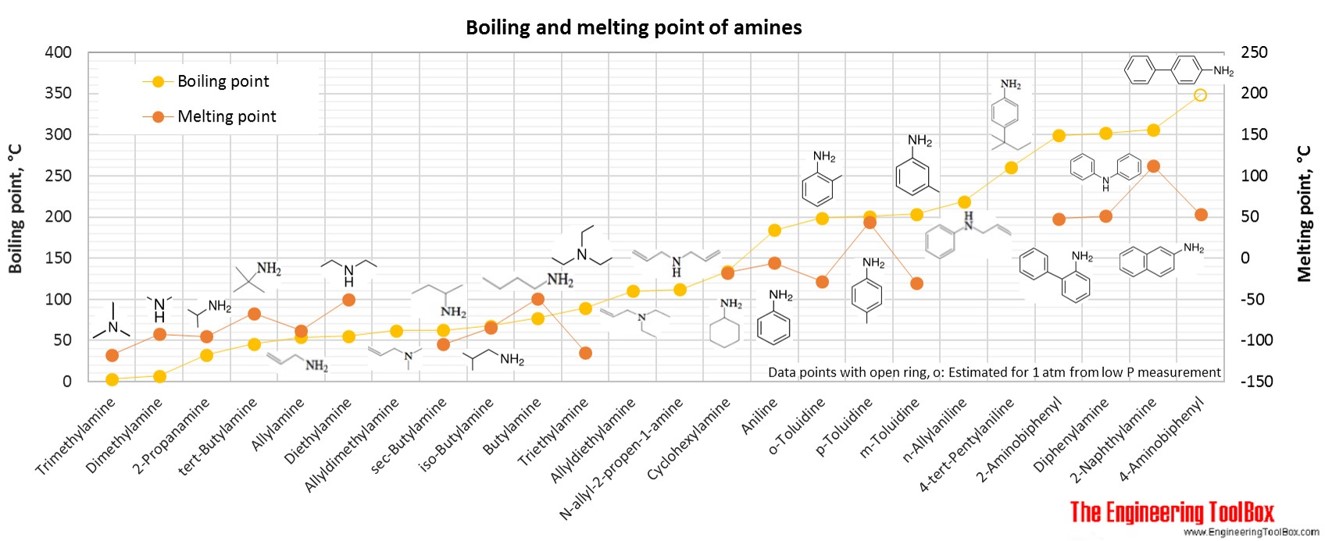 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It is used in the synthesis of choline tetramethylammonium hydroxide plant growth regulators or herbicides. That means that hydrogen bonding between tertiary amine molecules is impossible. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The major product will be the more substituted alkene that is the alkene with the most carbons directly attached to the alkene. CH 3 CO 2 H.
 Source: chegg.com
Source: chegg.com
The Flammable Range also called Explosive Range is the concentration range of a gas or vapor that will burn or explode if an ignition source is introduced. Previous year IIT JEE Fundamental Concepts Of Organic Chemistry Questions and answers are available. Flammable substance - fuel. As one might readily guess the inclusion of a heteroatom such as nitrogen in otherwise exclusively carbon and hydrogen molecules has quite an effect on the properties of amines as compared to alkanes. HOCH 2 CH 2 OH.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Methylamine and trimethylamine while liquids are easily vaporized. Water Solubility 1 2 and 3 amines can all form. H 2 NCH 2 CH 2 NH 2. Saltwater fish possess molecules called Trimethylamine N-oxide. Carboxylic acid Alcohols 12 Amines 3 AminesAlkanes.

The molecular weight and boiling point of compounds are directly related with the flavor development eg esters and aldehyde have higher molecular weight but lower boiling point but lactones have relatively high boiling point. It is a tertiary amine and a member of methylamines. Water Solubility 1 2 and 3 amines can all form. Std enthalpy of. Boiling Point and Dipole-Dipole Interactions.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point trimethylamine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.