Boiling point range of cyclohexane
Home » datasheet » Boiling point range of cyclohexaneBoiling point range of cyclohexane
Boiling Point Range Of Cyclohexane. Toxic by skin absorption and inhalation. Used to manufacture other chemicals especially dyes photographic chemicals agricultural chemicals and others. However polarity as seen the most important factor in adsorption chromatography. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention.
The wide range of boiling temperatures makes recovery and re-use difficult. In general a boiling point range of 1-2 C is usually taken as an indication of a pure material. The SN2 Reaction Mechanism. Solubility of Various Solutes at Room Temperature and Hot Temperatures Solute Unknown A Benzoic Acid Acetanilide Naphthalene Resorcinol Water Room Insoluble Insoluble Insoluble Insoluble. Toxic by skin absorption and inhalation. National Toxicology Program Chemical Repository Database.
Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals.
Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Cyclohexane iso-octanol or mixtures of these solvents. These mixtures when present at specific concentrations usually distill at a constant. The majority of solvent cleaning work is performed in equipment of two types relative to flash point either an open tank or a closed tank. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Denser than water 85 lb gal and slightly soluble in water.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
In increasing order of polarity Petroleum ether carbon tetrachloride cyclohexane ether acetone benzene toluene esters water etc It can b e used in. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. Having gone through the two different types of substitution reactions and talked about nucleophiles and electrophiles were finally in a position to reveal the mechanism for one of the most important reactions in organic chemistry. Melting point ºC Boiling point ºC Dielectric Constant Molecular Weight Acetic Acid-d 4 1165 1 204 5 22 17899 1 200 7 20 115 112 167 118 61 6408 Acetone-d 6 205 5 22 20668 1 2992 7 09 194 28 087 -94 565 207 6412 Acetonitrile-d 3 194. It may be necessary to add an additional low boiling point fraction to the crude oil mixture eg cyclohexane to obtain something below 70 C.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
This is because it is difficult to get clean and it. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. The majority of solvent cleaning work is performed in equipment of two types relative to flash point either an open tank or a closed tank. Cyclohexane iso-octanol or mixtures of these solvents. Most pure organic compounds melt over a narrow temperature range of 1-2 C.

158 C at 10 torr. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. Lipids with little or no polar groups triacylglycerides cholesterol. Toxic by skin absorption and inhalation. There is a wide range of fuel cell concepts that have entered trials.

Information gathered from the Merck Index Fourteenth Edition. Cyclohexane iso-octanol or mixtures of these solvents. 4 esters are highly soluble in hexanes. The solvents should also have sufficiently low boiling points to permit ready recovery of eluted material. Hawleys Condensed Chemical Dictionary 15th Edition.
 Source: researchgate.net
Source: researchgate.net
The SN2 Reaction Mechanism. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. The solvents should also have sufficiently low boiling points to permit ready recovery of eluted material. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. If it is done regularly it is probably best to keep sets of apparatus apart from the thermometer and watch glasses dedicated to the experiment.

The melting point range is defined as the span of temperature from the point at which the crystals first begin to liquefy to the point at which the entire sample is liquid. Solubility of lipids in solvents is based on the relative proportion of polar and non-polar groups in the matrix. RATE Hexane 561 0675 65-70 149-158 Cyclohexane 653 0784 80-82 176-180 0 55 65 0 55 Heptane 579 0695 92-100 198-212 15 30 146 01 45 VM P Naphtha 620 0744 119-141 256-285 50 32 153. Solvent Physical State Color Melting Point C Boiling Point C Benzoic Acid Solid White 122 249 Acetanilide Solid White 114 304 Naphthalene NA NA 805 218 Resorcinol Solid White 110-113 280 Table 2. Producing two immiscible liquid phases such as the example below with the addition of.
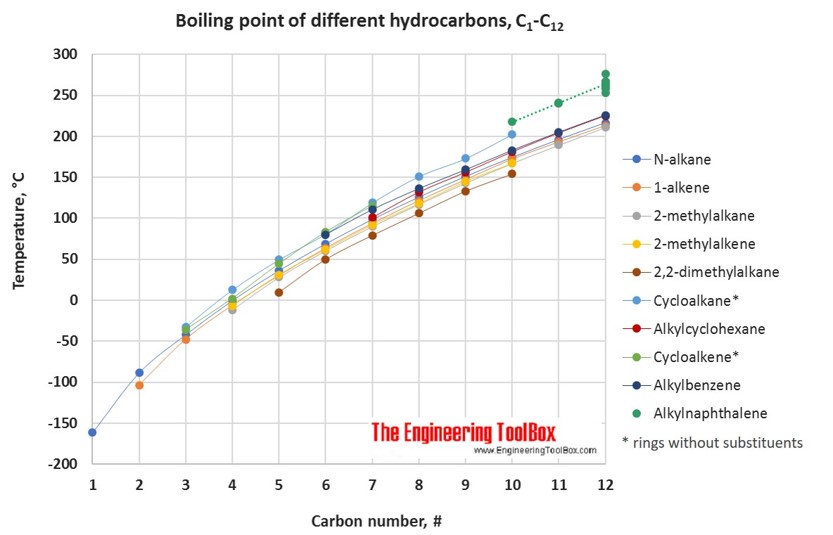 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Temperature K A B C Reference Comment. Solubility of lipids in solvents is based on the relative proportion of polar and non-polar groups in the matrix. You will use both of these properties later in the semester to identity an unknown liquid. Produces toxic oxides of nitrogen during combustion. Hawleys Condensed Chemical Dictionary 15th Edition.

Solubility of lipids in solvents is based on the relative proportion of polar and non-polar groups in the matrix. Hessel and Geiseler 1965. Determine the useful liquid range of the solvents. Producing two immiscible liquid phases such as the example below with the addition of. If it is done regularly it is probably best to keep sets of apparatus apart from the thermometer and watch glasses dedicated to the experiment.

158 C at 10 torr. Solubility of Various Solutes at Room Temperature and Hot Temperatures Solute Unknown A Benzoic Acid Acetanilide Naphthalene Resorcinol Water Room Insoluble Insoluble Insoluble Insoluble. 4 esters are highly soluble in hexanes. Solubility of lipids in solvents is based on the relative proportion of polar and non-polar groups in the matrix. Cyclohexane iso-octanol or mixtures of these solvents.

PRODUCT 60 F 6060 F C F F TCC KB PT. Its called the S N 2 reaction and its going to be extremely useful for us going forward. Chemical Resistance of Rubbers and Elastomers - Rsistance to chemicals. The potential for ignition of solvents in cold cleaning under shipment or storage conditions is assessed by measuring flash point in an open cup tester. The filters are produced with standard round threads according to STANAG 4155 EN 148- 1- Rd 40x17.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point range of cyclohexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.