Boiling point of xylenes
Home » datasheet » Boiling point of xylenesBoiling point of xylenes
Boiling Point Of Xylenes. Xylenes are highly soluble in blood and fat and are distributed widely in. The flash point of an oil product can be determined by several methods depending on the oil product and the quantity available. 8 hPa Xylene 20C Vapour density. Det er mine desorption efficiency DE at least once for each batch of charcoal used for sampling in the.
 Xylene And Its Export Kia Export From kiaexport.com
Xylene And Its Export Kia Export From kiaexport.com
Additionally if the liquid collection unit is not 100 effective some light volatile could be in the gaseous stream such as pentane benzene toluene xylenes and acetaldehyde. Liquid m-xylene is well absorbed through the skin but dermal absorption of m-xylene vapor up to 600 ppm does not appear to be appreciably absorbed. The odor of xylene is detectable at concentrations as low as 008 to 37 ppm parts of xylene per million parts of air and can be tasted in water at 053 to 18 ppm. Benzene toluene and xylenes can be made by various processes. Polyolefin melting point. METHOD 1501 Issue 3 dated 15 M arch 2003 - page 3 of 7 NIOSH Manual of Analytical Methods NMAM Fourth Edition 9.
2-propanol 30 35 99 acetone Acetonitrile acid alcohol alcohol extraction alcohol for labs alconox boiling point butyl alcohol calibration california cannabis cannabis extraction cgm devices chemical reactions chemicals chemicals for laboratories citronox cleaning cleaning agents cleaning alcohol cleaning electronics cleaning ppe products cleaning solvent.
However most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery. Lower viscosity products including light fuel oils and most fresh crudes are measured by the Tag closed-cup method. Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 12 carbon atoms and typically produces a reformate product containing C 6 to. The odor of xylene is detectable at concentrations as low as 008 to 37 ppm parts of xylene per million parts of air and can be tasted in water at 053 to 18 ppm. Insoluble in water Soluble in. 2-propanol 30 35 99 acetone Acetonitrile acid alcohol alcohol extraction alcohol for labs alconox boiling point butyl alcohol calibration california cannabis cannabis extraction cgm devices chemical reactions chemicals chemicals for laboratories citronox cleaning cleaning agents cleaning alcohol cleaning electronics cleaning ppe products cleaning solvent.
 Source: en.wikipedia.org
Source: en.wikipedia.org
7 Though accurate the Tag method uses a comparatively large volume of oil 50 to 70 mL. Xylene dimethylbenzene is used as a high boiling temperature solvent so that the reaction will proceed quickly. However most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery. Anthracene Maleic anhydride 910-dihydroanthracene- -910-αβ-succinic anhydride OBJECTIVES In this experiment you will Synthesize 910-dihydroanthracene-910-αβ-succinic anhydride. Since pyrolysis gas contains significant amount of carbon dioxide along with methane and some other combustible gases it might be used as a fuel for industrial combustion purposes 33.
 Source: researchgate.net
Source: researchgate.net
PT Po ACA Po BCB If B is the nonvolatile component then Po B 0 and the second term contributes nothing to the total vapor pressure. Formaldehyde is a potent irritant with an Immediately Dangerous to Life or Health. The boiling point is increased because the nonvolatile solid does not contribute to the total vapor pressure but it decreases the mole fraction of the liquid. A higher temperature is for the vapor pressure to equal the surrounding pressure. Separation Process Principles- Chemical and Biochemical Operations 3rd Edition.
 Source: chemsynthesis.com
Source: chemsynthesis.com
The boiling point for each isomer is around 140 C 284 F. Separation Process Principles- Chemical and Biochemical Operations 3rd Edition. Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 12 carbon atoms and typically produces a reformate product containing C 6 to. 23C 734F Evaporation rate. Liquid m-xylene is well absorbed through the skin but dermal absorption of m-xylene vapor up to 600 ppm does not appear to be appreciably absorbed.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This follows ASTM method D 1310. Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 12 carbon atoms and typically produces a reformate product containing C 6 to. 8 hPa Xylene 20C Vapour density. Xylenes o- m- p- Isomers 1330-20-7 25-10 GHS02-GHS07 H226-315-319-332 Dimethyl Carbonate 616-38-6 25-10 GHS02 H225 Naphtha Petroleum Hydrotreated Light 64742-49-0 25-10 GHS08 H304 Carbon Black 1333-86-4 10-25 Not Available Not Available Propylene Glycol Monobutyl Ether 5131-66-8 10-25 GHS07 H302-315-319 Ethylbenzene 100-41-4 10-25 GHS02-GHS07-GHS08 H225-304-332-351-373. 2-Xylene which has the highest boiling point is separated as the bottom distillate.
 Source: researchgate.net
Source: researchgate.net
PT Po ACA Po BCB If B is the nonvolatile component then Po B 0 and the second term contributes nothing to the total vapor pressure. Since pyrolysis gas contains significant amount of carbon dioxide along with methane and some other combustible gases it might be used as a fuel for industrial combustion purposes 33. Xylenes o- m- p- Isomers 1330-20-7 25-10 GHS02-GHS07 H226-315-319-332 Dimethyl Carbonate 616-38-6 25-10 GHS02 H225 Naphtha Petroleum Hydrotreated Light 64742-49-0 25-10 GHS08 H304 Carbon Black 1333-86-4 10-25 Not Available Not Available Propylene Glycol Monobutyl Ether 5131-66-8 10-25 GHS07 H302-315-319 Ethylbenzene 100-41-4 10-25 GHS02-GHS07-GHS08 H225-304-332-351-373. Det er mine desorption efficiency DE at least once for each batch of charcoal used for sampling in the. Xylenes isomers and mixture 1330207 o-Xylenes 95476 m-Xylenes 108383 p-Xylenes 106423 Antimony Compounds 0 Arsenic Compounds inorganic including arsine 0 Beryllium Compounds 0 Cadmium Compounds 0 Chromium Compounds 0 Cobalt Compounds 0 Coke Oven Emissions 0 Cyanide Compounds 1 0 Glycol ethers2 Modified1 0 1 EPA remove the compound ethylene glycol monobutyl.
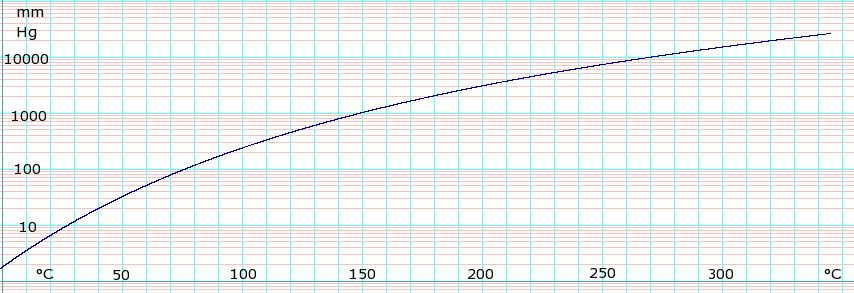 Source: en.wikipedia.org
Source: en.wikipedia.org
Xylenes are highly soluble in blood and fat and are distributed widely in. Xylene dimethylbenzene is used as a high boiling temperature solvent so that the reaction will proceed quickly. 8 hPa Xylene 20C Vapour density. Top 5 Uses of Xylenes C8H10. Additionally if the liquid collection unit is not 100 effective some light volatile could be in the gaseous stream such as pentane benzene toluene xylenes and acetaldehyde.
 Source: kiaexport.com
Source: kiaexport.com
Xylene dimethylbenzene is used as a high boiling temperature solvent so that the reaction will proceed quickly. 2-propanol 30 35 99 acetone Acetonitrile acid alcohol alcohol extraction alcohol for labs alconox boiling point butyl alcohol calibration california cannabis cannabis extraction cgm devices chemical reactions chemicals chemicals for laboratories citronox cleaning cleaning agents cleaning alcohol cleaning electronics cleaning ppe products cleaning solvent. Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 12 carbon atoms and typically produces a reformate product containing C 6 to. This follows ASTM method D 1310. Polyolefin melting point.

Benzene toluene and xylenes can be made by various processes. PT Po ACA Po BCB If B is the nonvolatile component then Po B 0 and the second term contributes nothing to the total vapor pressure. Formaldehyde is a potent irritant with an Immediately Dangerous to Life or Health. 1atm 1 atm 760 mmHg IDLH. Here Molecular Weight characterization needs can sometimes be met with simple Ubbelohde intrinsic viscosity data or melt flow index determinations.
 Source: researchgate.net
Source: researchgate.net
Lower viscosity products including light fuel oils and most fresh crudes are measured by the Tag closed-cup method. Additionally if the liquid collection unit is not 100 effective some light volatile could be in the gaseous stream such as pentane benzene toluene xylenes and acetaldehyde. 8 hPa Xylene 20C Vapour density. Top 5 Uses of Xylenes C8H10. Liquid m-xylene is well absorbed through the skin but dermal absorption of m-xylene vapor up to 600 ppm does not appear to be appreciably absorbed.

Here Molecular Weight characterization needs can sometimes be met with simple Ubbelohde intrinsic viscosity data or melt flow index determinations. 2-propanol 30 35 99 acetone Acetonitrile acid alcohol alcohol extraction alcohol for labs alconox boiling point butyl alcohol calibration california cannabis cannabis extraction cgm devices chemical reactions chemicals chemicals for laboratories citronox cleaning cleaning agents cleaning alcohol cleaning electronics cleaning ppe products cleaning solvent. 2 Organic Chemistry with Vernier. Det er mine desorption efficiency DE at least once for each batch of charcoal used for sampling in the. Here Molecular Weight characterization needs can sometimes be met with simple Ubbelohde intrinsic viscosity data or melt flow index determinations.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of xylenes by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.