Boiling point of water ethylene glycol mixture
Home » datasheet » Boiling point of water ethylene glycol mixtureBoiling point of water ethylene glycol mixture
Boiling Point Of Water Ethylene Glycol Mixture. Common base fluids include water ethylene glycol and oil. The charge on the surface was about 33 pC. -GPSA Engineering Data Book Gas Processing 12th ed. Ethylene oxide is a flammable gas with a somewhat sweet odor.
 Freezing And Boiling Temperatures Of Various Fluids Download Table From researchgate.net
Freezing And Boiling Temperatures Of Various Fluids Download Table From researchgate.net
Freezing-point depression is a drop in the temperature at which a substance freezes caused when a smaller amount of another non-volatile substance is added. Biomed Appl 6192251-257Kenyon AS Shi X Wang Y Ng WH Prestridge R Sharp K 1998. Since the density closely matches the known value we conclude that this is an authentic sample of ethylene glycol. However the boiling point of deionized water can be pushed up by mixing it with anti-freezing materials like ethylene glycol EG. Nanofluids have novel properties that make them potentially useful in many applications in heat transfer including microelectronics fuel cells pharmaceutical processes and hybrid-powered engines engine coolingvehicle thermal management domestic refrigerator chiller heat exchanger in grinding. So while providing freeze protection and an increased boiling point ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water.
This coolant has a boiling point of 375 degrees F and will not vaporize–eliminating overheating boil-over and after-boil.
Before you service with Evans your water. Vapour Pressure 130148 61 Vapour Pressure and Boiling Point 130 611 Vapour Pressure 130 612 Boiling Point 131 62 Phase Behaviour of Pure Substances 131 63 Vapour Pressure and Temperature 133 631 The Clapeyron Equation 133 632 The ClausiusClapeyron Equation 134 633 The Antoine Equation 135 64 Vapour Pressure Plots 137 641 Equal-Temperature Reference-Substance Plots 138. What is the freezing point of water at 5000 meters altitude What is the freezing point of water at 5000 meters altitude email protected. Melting point degrees Celsius. However the boiling point of deionized water can be pushed up by mixing it with anti-freezing materials like ethylene glycol EG. 1 C 1 C tc TK 273 K 5800 K 273 K.
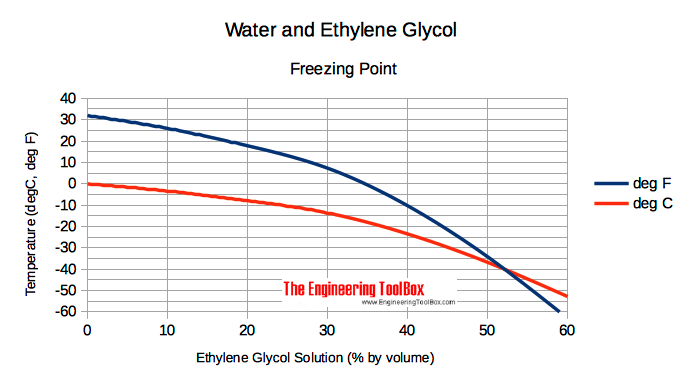 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Rotary Recuperator Consists Heat Wheel - of a revolving cylinder divided into segments packed with coarsely-knitted. What is Hydrochloric Acid. All matter can be classified as either a pure substance. Each substance in the mixture retains its own set of chemical and physical properties. Biomed Appl 6192251-257Kenyon AS Shi X Wang Y Ng WH Prestridge R Sharp K 1998.
 Source: researchgate.net
Source: researchgate.net
Common base fluids include water ethylene glycol and oil. Sep 23 2018 Interestingly the boiling point and melting point of a substance can give us an indication of how pure it is. The formation of large. The low vapor pressure reduces stress on the engine cooling system components. A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical.
 Source: researchgate.net
Source: researchgate.net
Simple at-site detection of diethylene glycolethylene glycol contamination of glycerin and glycerin- based raw. Since the density closely matches the known value we conclude that this is an authentic sample of ethylene glycol. Studied the boiling heat transfer characteristics of GNP nanofluids with different mass concentrations using ethylene glycol and DI water with a volume ratio of 6040 as the base fluid and found that at a low concentration 002 the deposition of nanoparticles increased the surface wettability which significantly improved the boiling characteristic of the base liquid. So while providing freeze protection and an increased boiling point ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water. The higher temperature of the extract air transfer heat to a waterethylene glycol mix in the interconnecting pipework 50 - 60 efficient.
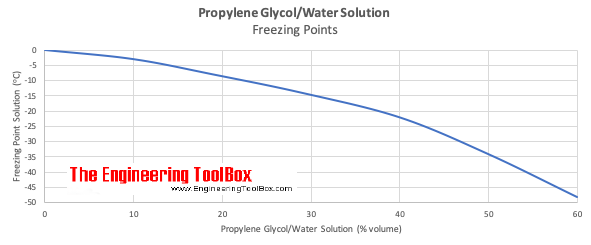 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Oxygen sulphur iron etc. Examples include adding salt into water used in ice cream makers and for de-icing roads alcohol in water ethylene or propylene glycol in water used in antifreeze in cars adding copper to molten silver used to make solder that. Sep 23 2018 Interestingly the boiling point and melting point of a substance can give us an indication of how pure it is. A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three quarters that of pure water thus requiring increased flow rates in same-system comparisons with water. How Do We Classify Matter.
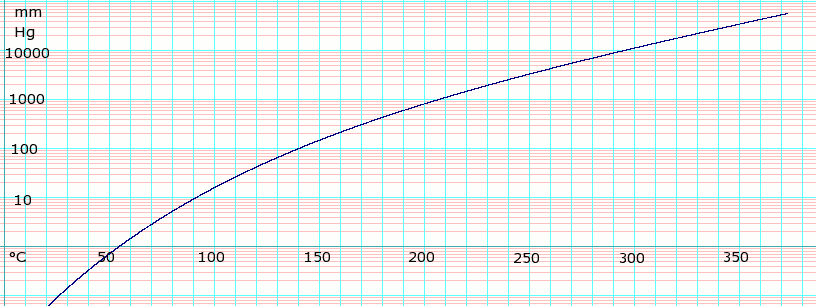 Source: en.wikipedia.org
Source: en.wikipedia.org
Boiling point degrees Celsius. 45359 g 1 ft gmL 6922 lbft 1 lb 3048 cm 3. This coolant has a boiling point of 375 degrees F and will not vaporize–eliminating overheating boil-over and after-boil. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. It is a by-product of chlorine manufacture along with sodium hypochlorite.
 Source: processecology.com
Source: processecology.com
Freezing-point depression is a drop in the temperature at which a substance freezes caused when a smaller amount of another non-volatile substance is added. Melting point degrees Celsius. The formation of large. Decide whether each of the following are mixtures or pure substances. Studied the boiling heat transfer characteristics of GNP nanofluids with different mass concentrations using ethylene glycol and DI water with a volume ratio of 6040 as the base fluid and found that at a low concentration 002 the deposition of nanoparticles increased the surface wettability which significantly improved the boiling characteristic of the base liquid.

What is Hydrochloric Acid. Simple at-site detection of diethylene glycolethylene glycol contamination of glycerin and glycerin- based raw. How Do We Classify Matter. Examples include adding salt into water used in ice cream makers and for de-icing roads alcohol in water ethylene or propylene glycol in water used in antifreeze in cars adding copper to molten silver used to make solder that. A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three quarters that of pure water thus requiring increased flow rates in same-system comparisons with water.
 Source: forum.miata.net
Source: forum.miata.net
Nanofluids have novel properties that make them potentially useful in many applications in heat transfer including microelectronics fuel cells pharmaceutical processes and hybrid-powered engines engine coolingvehicle thermal management domestic refrigerator chiller heat exchanger in grinding. Sep 23 2018 Interestingly the boiling point and melting point of a substance can give us an indication of how pure it is. 45359 g 1 ft gmL 6922 lbft 1 lb 3048 cm 3. A circulating pump and pressurisation provisions are required for the pipework system. How Do We Classify Matter.
 Source: sciencedirect.com
Source: sciencedirect.com
Before you service with Evans your water. The formation of large. Evans Cooling high performance waterless engine coolant will also eliminate corrosion and offers protection to temperatures as low as -40 degrees F. A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three quarters that of pure water thus requiring increased flow rates in same-system comparisons with water. Freezing-point depression is a drop in the temperature at which a substance freezes caused when a smaller amount of another non-volatile substance is added.
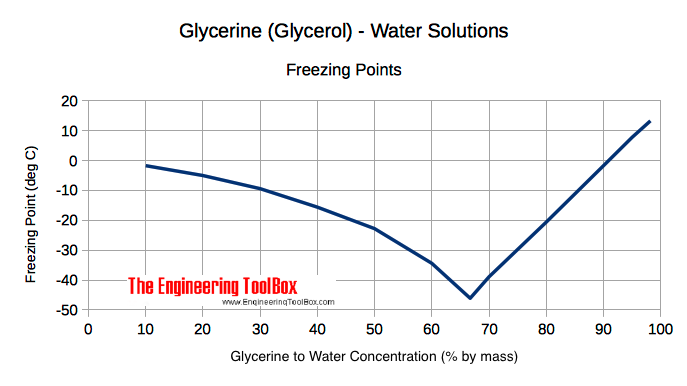 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Simultaneous determination of ethylene glycol propylene glycol 13-butylene glycol and 23-butylene glycol in human serum and urine by wide-bore column gas chromatography. 45 Upon injection into the levitator the droplet took a spherical shape with a radius of 58 μm. 118 20 degrees Celsius. The charge on the surface was about 33 pC. 1 C 1 C tc TK 273 K 5800 K 273 K.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of water ethylene glycol mixture by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.