Boiling point of sodium thiosulfate
Home » datasheet » Boiling point of sodium thiosulfateBoiling point of sodium thiosulfate
Boiling Point Of Sodium Thiosulfate. Chemical Properties of Sodium Sulfite. Hence it is used in cooling a fast reactor. 2-8-1 or 1s 2 2s 2 2p 6 3s 1. Contain 155 moles of sodium thiosulfate.
 Sodium Thiosulfate Wikipedia From en.wikipedia.org
Sodium Thiosulfate Wikipedia From en.wikipedia.org
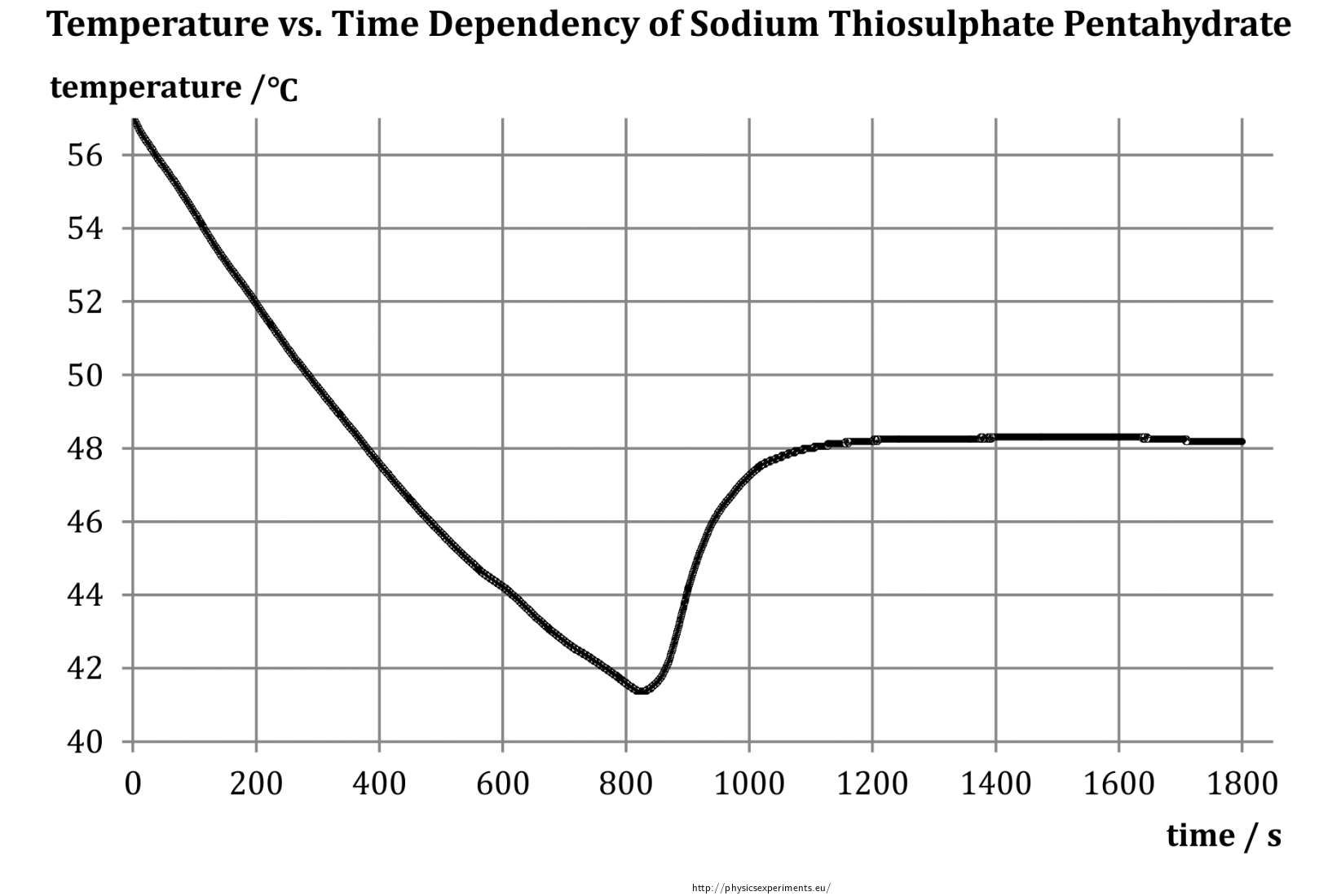
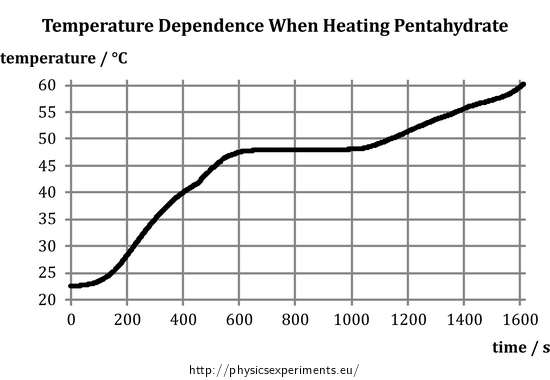
Crystallisation occurs when the solution solvent evaporates and the concentration of the solute reaches saturation point. 0971 20 C oxidation states 1 1 rare electron configuration. This solution is stored in a refrigerator. With two sets of apparatus. Add the sodium thiosulfate pentahydrate weigh precisely with a margin of 001 g o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask. In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form applying electrolysis to fused sodium.
This method is much cheaper than that of electrolyzing sodium hydroxide as was used several years ago.
4446 C 832 F density at 20 C 68 F rhombic. Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus. Standardization of sodium thiosulphate. Sort by Relevance. The solubility of sodium thiosulfate in water is 701g100 mL at 20 o C and 231g100mL at 100 o. At this stage the solute begins to precipitate out of solution.

Making Dilutions Diluting a solution reduces the number of moles. The leaching process is performed in a series of vats each having a capacity of ca. Crystallisation occurs when the solution solvent evaporates and the concentration of the solute reaches saturation point. A similar activity suitable for a student practical is freezing super-cooled sodium thiosulfate. The reactors operating temperature is much less than the boiling point of sodium.
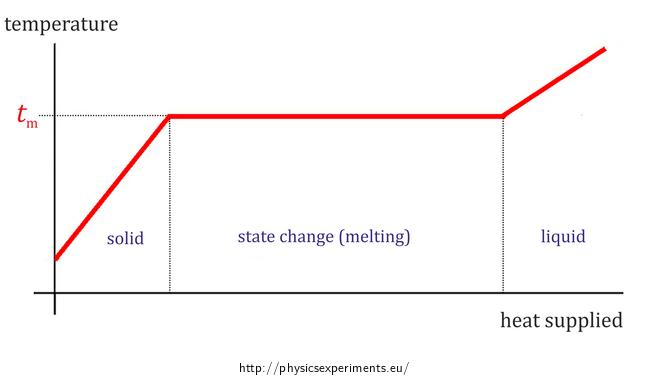
 Source: physicsexperiments.eu
Source: physicsexperiments.eu
Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus. The solubility of sodium thiosulfate in water is 701g100 mL at 20 o C and 231g100mL at 100 o. Design Strategies - Piping systems and design strategies - documentation PID flow. Use the clear supernate. Iodine that crystallises can be reused but any remaining deposits can be cleaned by soaking the equipment in a bath of 1 M sodium thiosulfate.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Upon contact with strong or weak acids Na 2 SO 3. Crystallisation occurs when the solution solvent evaporates and the concentration of the solute reaches saturation point. Typically it is available as the white or colorless pentahydrate Na 2 S 2 O 3 5H 2 OThe solid is an efflorescent loses water readily crystalline substance that dissolves well in water. Because sodium is extremely reactive it never occurs in the free state in Earths crust.
 Source: physicsexperiments.eu
Source: physicsexperiments.eu
Sodium thiosulfate pentahydrate-X— XXXX-Sodium thiosulfate XX-231-867-5- XXXXX Legend. Add one gram of starch to few ml of water prepare slurry and add gradually to 100 ml of boiling water till a translucent solution will be obtained. Add the sodium thiosulfate pentahydrate weigh precisely with a margin of 001 g o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask. Various industries including petroleum chemicals soaps textiles and paper have been using sodium compounds on a large-scale to carry out their important processes. For individuals with significant inhalation exposure monitor arterial blood gases.
 Source: sciencemadness.org
Source: sciencemadness.org
The solubility of sodium thiosulfate in water is 701g100 mL at 20 o C and 231g100mL at 100 o. The density of sodium thiosulfate corresponds to 1667 grams per cubic centimetres. The underflow of each vat is heated before passing to the next one since the dissolution of sodium nitrate is endothermic. Warm 40 - 45 C mother liquor recycled to leaching contains ca. NaNO 3 di- and tri-sodium phosphates sodium thiosulfate Na 2 S 2 O 3 5H 2 O and borax Na 2 B 4 O 7 10H 2 O.
 Source: physicsexperiments.eu
Source: physicsexperiments.eu
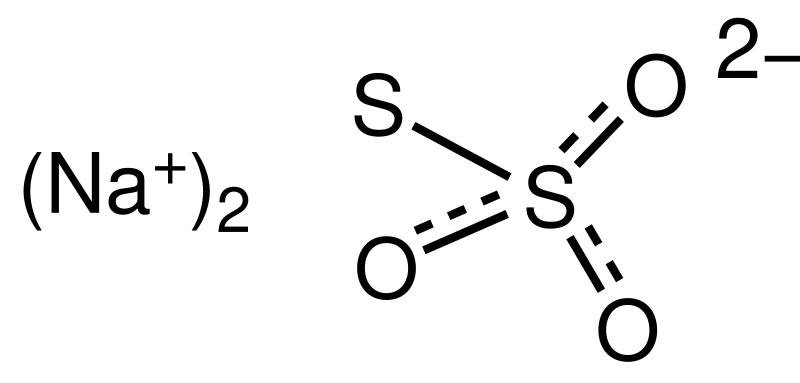
Sodium thiosulfate sodium thiosulphate is an inorganic compound with the formula Na 2 S 2 O 3. Dissolve 45 g Na2S2O35H2O and 25g sodium borate Na2B4O7 reagent grade for a preservative in 1 liter of distilled water. Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus. With two sets of apparatus. The underflow of each vat is heated before passing to the next one since the dissolution of sodium nitrate is endothermic.
Sodium thiosulfate pentahydrate-X— XXXX-Sodium thiosulfate XX-231-867-5- XXXXX Legend. Pour this emulsion into 1 L of boiling water allow to boil a few minutes and let settle overnight. The reactors operating temperature is much less than the boiling point of sodium. Do not administer neutralizing substances since the resultant exothermic reaction could further damage tissue. 0971 20 C oxidation states 1 1 rare electron configuration.
 Source: physicsexperiments.eu
Source: physicsexperiments.eu
Sodium Thiosulfate Stock solution 018 M. For individuals with significant inhalation exposure monitor arterial blood gases. SOLUTION OF SODIUM THIOSULFATE 01 moll In the volumetric lask. Design Strategies - Piping systems and design strategies - documentation PID flow. Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus.
 Source: chemistrypage.in
Source: chemistrypage.in
Contain 155 moles of sodium thiosulfate. It is the most abundant of the alkali group of metals. Warm 40 - 45 C mother liquor recycled to leaching contains ca. Add one gram of starch to few ml of water prepare slurry and add gradually to 100 ml of boiling water till a translucent solution will be obtained. In compounds sodium is usually ionically bonded to water and anions and is viewed as a hard Lewis acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Uses of Sodium Compounds. The underflow of each vat is heated before passing to the next one since the dissolution of sodium nitrate is endothermic. UOP621-98 Boiling Point Distribution of Hydrocarbons by Gas Chromatography. At this stage the solute begins to precipitate out of solution. Add the sodium thiosulfate pentahydrate weigh precisely with a margin of 001 g o Add distilled water to ill up the quantity to the correct volume o Close the volumetric lask.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of sodium thiosulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.