Boiling point of sodium sulfate
Home » datasheet » Boiling point of sodium sulfateBoiling point of sodium sulfate
Boiling Point Of Sodium Sulfate. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. 270 g100 mL water 20 C Solubility. To learn more about the Structure Properties Preparations Uses and FAQs of Sodium sulphate Visit BYJUS for detailed information. Properties and production.
 Sodium Sulfate From cs.mcgill.ca
Sodium Sulfate From cs.mcgill.ca
Lead has a higher melting point 3275C and significantly higher boiling point 1750C compared to that of sodium which significantly impacts on the reactor. 05 ppm SO2 Solubility in Water. Sodium hydroxide is also the most common base used in chemical laboratories. Boiling point C 886 1413 De- composes Density at 20 C gcm3 071 217 253 Vapour pressure kPa 0133 Water solubility at 0 C gl reacts violently 357 71 infinitely soluble soluble Organoleptic properties The taste threshold for sodium in water depends on the associated anion and the temperature of the solution. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Sodium Sulfate Na2SO4 -Sodium sulfate is the sodium salt of sulfuric acid.
Not Applicable Coefficient of WaterOil Distribution.
88 sodium 4-dodeocylbenezenesulfonate sodium salt has a molecular weight of 348477 gramsmole. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. Properties and production. Substance Substance name. Sodium Lauryl Sulfate Sodium dodecyl sulfate 151-21-3.
 Source: vias.org
Source: vias.org
Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. It is mainly used as a filler in the manufacture of powdered home laundry. Identification Product form. Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Soluble in glycerol Insoluble in ammonia chlorine.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
Its a soft metal reactive and with a low. Not Applicable Odor Threshold. Energy of first ionisation. Sodium Sulfate Anhydrous Safety Data Sheet according to Federal Register Vol. Boiling point C 886 1413 De- composes Density at 20 C gcm3 071 217 253 Vapour pressure kPa 0133 Water solubility at 0 C gl reacts violently 357 71 infinitely soluble soluble Organoleptic properties The taste threshold for sodium in water depends on the associated anion and the temperature of the solution.
 Source: coleparmer.co.uk
Source: coleparmer.co.uk
Boiling point - the temperature at which a liquid turns into a gas. Also it is a more inert liquid metal than sodium. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 334 C 921 F.
 Source: en.wikipedia.org
Source: en.wikipedia.org
3065 K dehydration of heptahydrate 500 C anhydrous Boiling point. Electronic shell Ne 3s 1. Melting point - the temperature at which a solid turns into a liquid. Sodium Sulfate Na2SO4 -Sodium sulfate is the sodium salt of sulfuric acid. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The pentahydrate of this salt has a melting point of 3214 K and a boiling point of 373 K. Decomposes Solubility in water. Sodium hydroxide is also the most common base used in chemical laboratories. In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form applying electrolysis to fused. 05 ppm SO2 Solubility in Water.
Electronic shell Ne 3s 2 3p 4. However it dissociates in water and some other polar solvents to yield. Lead has a higher melting point 3275C and significantly higher boiling point 1750C compared to that of sodium which significantly impacts on the reactor. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Energy of first ionisation.

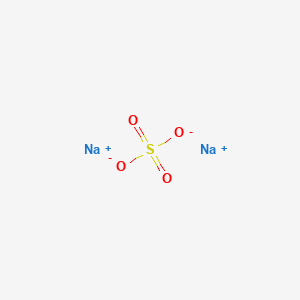
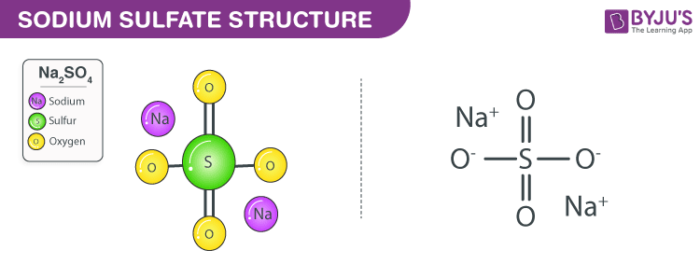
270 g100 mL water 20 C Solubility. Identification of the substancemixture and of the supplier Product name. Electronic shell Ne 3s 1. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Sodium sulfate also known as sodium sulphate or sulfate of soda is the inorganic compound with formula Na 2 SO 4 as well as several related hydratesAll forms are white solids that are highly soluble in water.
 Source: byjus.com
Source: byjus.com
Corrosion - Corrosion in piping systems - caused by thermodynamic and electrochemical processes - corrosion problems and methods of protection and prevention. Sulphur is a multivalent non-metal abundant tasteless. Sodium SulfateAnhydrous ManufacturerSupplier Trade name. 137 at 20C Evaporation Rate. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
Electronic shell Ne 3s 2 3p 4. Sodium Sulfate Na2SO4 -Sodium sulfate is the sodium salt of sulfuric acid. 0971 20 C oxidation states 1 1 rare electron configuration. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. To learn more about the Structure Properties Preparations Uses and FAQs of Sodium sulphate Visit BYJUS for detailed information.
 Source: trc-canada.com
Source: trc-canada.com
Not Applicable Odor Threshold. The ICSC project is a common undertaking between the World Health Organization WHO and. Identification of the substancemixture and of the supplier Product name. This material is stable and not considered reactive under normal temperatures and pressures. SDBS boiling point is above the temperature for its decomposition.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of sodium sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.