Boiling point of sodium nitrate
Home » datasheet » Boiling point of sodium nitrateBoiling point of sodium nitrate
Boiling Point Of Sodium Nitrate. After treating many gallons of water the resin will run out of chloride. Its a soft metal reactive and with a low. The next step is to convert all other nitrates into nitrate of potash. For example suppose you have a near-boiling solution of potassium nitrate in water.
 Thermodynamic Representation Of Aqueous Sodium Nitrate And Nitric Acid Solution With Electrolyte Nrtl Model Sciencedirect From sciencedirect.com
Thermodynamic Representation Of Aqueous Sodium Nitrate And Nitric Acid Solution With Electrolyte Nrtl Model Sciencedirect From sciencedirect.com
The saltpeter ley liquid contains saltpeter as well as nitrate of lime and magnesia and chlorides of sodium and potassium. REG 10 ppm 0001 sodium nitrite - Alone as color fixative in smoked cured tuna -172175. Abundant sodium is found in the sun and stars. Melting point C 9783 801 851 Boiling point C 886 1413 De-composes Density at 20 C gcm3 071 217 253 Vapour pressure kPa 0133 Water solubility at 0 C gl reacts violently 357 71 infinitely soluble soluble Organoleptic properties The taste threshold for sodium in water depends on the associated anion and the temperature of the. Kills everything except some endospores. It is a source of nitrogen and nitrogen was named after niterPotassium nitrate is one of several nitrogen-containing compounds.
For example suppose you have a near-boiling solution of potassium nitrate in water.
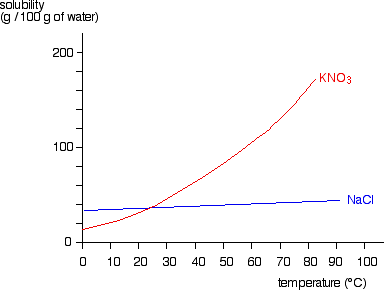
Melting point C 9783 801 851 Boiling point C 886 1413 De-composes Density at 20 C gcm3 071 217 253 Vapour pressure kPa 0133 Water solubility at 0 C gl reacts violently 357 71 infinitely soluble soluble Organoleptic properties The taste threshold for sodium in water depends on the associated anion and the temperature of the. Kills everything except some endospores. All the potassium. Regenerating the resin with a concentrated solution of sodium chloride you can use bicarbonate instead of chloride recharges it for further treatment. At all temperatures above that marked on the graph about 57C 100 g of water will dissolve more than 100 g of potassium nitrate. It is a source of nitrogen and nitrogen was named after niterPotassium nitrate is one of several nitrogen-containing compounds.

The more ash the stronger the water will be. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. Vice-Chair 2007 and Chair 2009 Gordon Research Conference on. Freezing point depression is a colligative property of matter. Kills everything except some endospores.
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. At all temperatures above that marked on the graph about 57C 100 g of water will dissolve more than 100 g of potassium nitrate. Sodium nitrite - PRES REG 100 ppm to 200 ppm - In loin muscle of smoked chub - 172177. For the purposes of purifying drinking water 100 o for five minutes is a standard in the mountains though there have been some reports that Giardia cysts can survive this process. This is done by taking lots of hardwood ashes and mixing them into a vat of water.
 Source: chemguide.co.uk
Source: chemguide.co.uk
Melting point - the temperature at which a solid turns into a liquid. After treating many gallons of water the resin will run out of chloride. Energy of third ionisation. Chair-Elect 2007 Chair 2008 Immediate Past Chair 2009 American Chemical Society Division of Chemical Education. Sodium nitrate was referred to as white gold in the 19th century.
 Source: sciencedirect.com
Source: sciencedirect.com
8829 C 1621 F specific gravity. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. 8829 C 1621 F specific gravity. For nitrate removal the resin exchanges chloride ions for nitrate and sulfate ions in the water. Sodium nitrate Revision Date 25-Apr-2019 9.
 Source: numerade.com
Source: numerade.com
For example suppose you have a near-boiling solution of potassium nitrate in water. Sir Humphrey Davy in 1807. Electronic shell of mercury Xe 4f 14 5d 10 6s 2. 011 nm 2 Isotopes or mercury. The more ash the stronger the water will be.

Energy of second ionisation. Physical and chemical properties Physical State Solid Crystalline Appearance White Odor Odorless Odor Threshold No information available pH 55-8 5 aq. It also inactivates viruses. Well take a solution containing 100 g of potassium nitrate and 100 g of water. Standard potential of mercury 0854 V Hg2 Hg Density of Mercury.

In 1807 Sir Humphry Davy became the first to prepare sodium in its elemental form applying electrolysis to fused sodium. Solution Melting PointRange 306 C 5828 F Boiling PointRange 380 C 716 F Flash Point No information available Evaporation Rate Not. Sir Humphrey Davy in 1807. Sodium nitrate is the chemical compound with the formula Na N O 3This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile to distinguish it from ordinary saltpeter potassium nitrateThe mineral form is also known as nitratine nitratite or soda niter. Figure 1 shows how the ion exchange process works.
 Source: wikiwand.com
Source: wikiwand.com
For the purposes of purifying drinking water 100 o for five minutes is a standard in the mountains though there have been some reports that Giardia cysts can survive this process. 11 and atomic weight 229898. Sodium is a good conductor of electricity which means that electric current can pass through this element without much resistance. The dominant yellow component in their light is the result of sodium atoms in a. Boiling point - the temperature at which a liquid turns into a gas.
 Source: sciencemadness.org
Source: sciencemadness.org
For example suppose you have a near-boiling solution of potassium nitrate in water. Vice-Chair 2007 and Chair 2009 Gordon Research Conference on. Both the melting and boiling point of sodium are quite high. For nitrate removal the resin exchanges chloride ions for nitrate and sulfate ions in the water. Boiling point - the temperature at which a liquid turns into a gas.
 Source: researchgate.net
Source: researchgate.net
Chair-Elect 2007 Chair 2008 Immediate Past Chair 2009 American Chemical Society Division of Chemical Education. Standard potential of mercury 0854 V Hg2 Hg Density of Mercury. It also inactivates viruses. Both the melting and boiling point of sodium are quite high. Colligative properties depend on the number of particles present not on the type of particles or their mass.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of sodium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.