Boiling point of sodium iodide
Home » datasheet » Boiling point of sodium iodideBoiling point of sodium iodide
Boiling Point Of Sodium Iodide. Boiling point - the temperature at which a liquid turns into a gas. Salt is iodized to help prevent cretinism in children as well as hypothyroidism and. 7295 JmolK Hydrogen Bond Acceptor. Its boiling point is at 883 C and melting point is at 9772 C.
With further heating above the 657 C boiling point the compound decomposes to Na 2 O releasing O 2. 7295 JmolK Hydrogen Bond Acceptor. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Abundant sodium is found in the sun and stars. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation. Take A Sneak Peak At The Movies Coming Out This Week 812 New Movie Releases This Weekend.
Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine.
Both the melting and boiling point of sodium are quite high. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. With further heating above the 657 C boiling point the compound decomposes to Na 2 O releasing O 2. Sodium oxide reacts with carbon dioxide to form. It is highly soluble in water with a lower solubility in polar solvents such as ethanol and methanol. The Boiling Point of an Iodine Solution Find the boiling point of a solution of 921 g of iodine I 2 in 8000 g of chloroform CHCl 3 assuming that the iodine is nonvolatile and that the solution is ideal.
 Source: en.wikipedia.org
Source: en.wikipedia.org
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. The iodine can be sublimed by mild heating. It may also be produced by passing ozone gas over solid sodium iodide inside a platinum or palladium tube. 7295 JmolK Hydrogen Bond Acceptor.
 Source: webelements.com
Source: webelements.com
The platinum or palladium catalyzes the reaction and is not attacked by the. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation. Quadratic Formula Circumference Formula Compound Interest Formula Midpoint Formula Arc Length Formula Area of a Triangle Formula Exponential Growth Formula Percent Change Formula Point-slope formula Simple Interest Formula. Solution We can solve this problem using four steps. Abundant sodium is found in the sun and stars.
 Source: byjus.com
Source: byjus.com
Iodized salt may also contain dextrose a sugar to stabilize the iodine. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Convert from grams to moles of I 2 using the molar mass of I. Physical Properties of Sodium Oxide Na 2 O. One of the most common additives is iodine in the form of potassium iodide sodium iodide or sodium iodate.
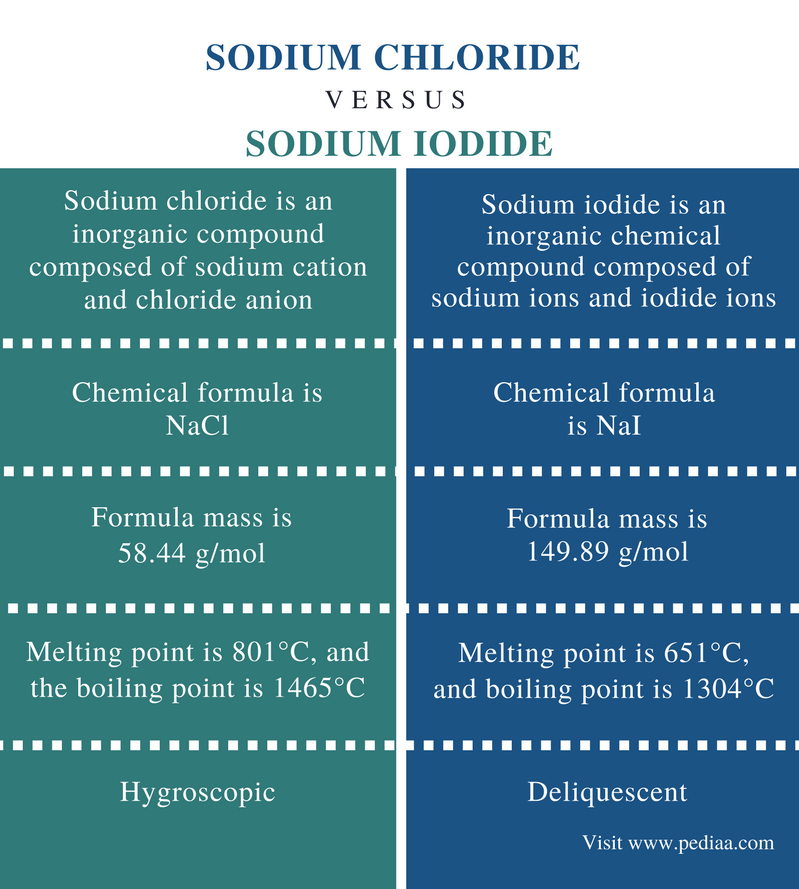 Source: pediaa.com
Source: pediaa.com
Pure sodium hydroxide is a colorless crystalline solid that melts at 318 C 604 F without decomposition and with a boiling point of 1388 C 2530 F. The ozone oxidizes the sodium to form sodium peroxide. The dominant yellow component. Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. It is highly soluble in water with a lower solubility in polar solvents such as ethanol and methanol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Click to see our best Video content. One of the most common additives is iodine in the form of potassium iodide sodium iodide or sodium iodate. Radiolabelled compound DB09293 is used as a diagnostic tool to evaluate thyroid function and morphology. Abundant sodium is found in the sun and stars. Both the melting and boiling point of sodium are quite high.

The ozone oxidizes the sodium to form sodium peroxide. Sodium is a good conductor of electricity which means that electric current can pass through this element without much resistance. 7295 JmolK Hydrogen Bond Acceptor. Both the melting and boiling point of sodium are quite high. Abundant sodium is found in the sun and stars.

Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Radiolabelled compound DB09293 is used as a diagnostic tool to evaluate thyroid function and morphology. Iodized salt may also contain dextrose a sugar to stabilize the iodine. Chemical Properties of Sodium Oxide Na 2 O.
 Source: scbt.com
Source: scbt.com
Salt is iodized to help prevent cretinism in children as well as hypothyroidism and. Click to see our best Video content. Convert from grams to moles of I 2 using the molar mass of I. Iodine deficiency is considered the biggest preventable cause of intellectual disability once known as mental retardation. The Boiling Point of an Iodine Solution Find the boiling point of a solution of 921 g of iodine I 2 in 8000 g of chloroform CHCl 3 assuming that the iodine is nonvolatile and that the solution is ideal.
 Source: pediaa.com
Source: pediaa.com
Solution We can solve this problem using four steps. It is highly soluble in water with a lower solubility in polar solvents such as ethanol and methanol. Its boiling point is at 883 C and melting point is at 9772 C. Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties. Quadratic Formula Circumference Formula Compound Interest Formula Midpoint Formula Arc Length Formula Area of a Triangle Formula Exponential Growth Formula Percent Change Formula Point-slope formula Simple Interest Formula.
 Source: lobachemie.com
Source: lobachemie.com
Radiolabelled compound DB09293 is used as a diagnostic tool to evaluate thyroid function and morphology. Sodium is a good conductor of electricity which means that electric current can pass through this element without much resistance. It may also be produced by passing ozone gas over solid sodium iodide inside a platinum or palladium tube. The iodine can be sublimed by mild heating. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of sodium iodide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.