Boiling point of sodium acetate
Home » datasheet » Boiling point of sodium acetateBoiling point of sodium acetate
Boiling Point Of Sodium Acetate. Ethylene vinyl acetate is a type of plastic a polymer with a wide variety of uses both residential and industrial. The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole-Dipole Van der Waals dispersion force. The Ethyl Acetate that is collected is. The crystal structure of anhydrous Na 2 SO 3 is hexagonal whereas the heptahydrate crystals have a monoclinic structure.
 Phase Diagram Of Sodium Acetate And Water A Vapor B Anhydrous Download Scientific Diagram From researchgate.net
Phase Diagram Of Sodium Acetate And Water A Vapor B Anhydrous Download Scientific Diagram From researchgate.net
The crystal structure of anhydrous Na 2 SO 3 is hexagonal whereas the heptahydrate crystals have a monoclinic structure. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Therefore ethyl acetate will be the first fraction collected as the distillate. Try to keep the stream of the. An example of a supercooled liquid can be made by heating solid sodium acetate trihydrate NaCH 3 CO 2 3 H 2 O. When this solid melts the sodium acetate dissolves in the water that was trapped in the crystal to form a solution.
The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding.
Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodine. Therefore ethyl acetate will be the first fraction collected as the distillate. Ethyl ethanoate ethyl acetate is an ester. 8829 C 1621 F specific gravity. Under standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions. 56 C 1328 F Boiling point of alcohol.
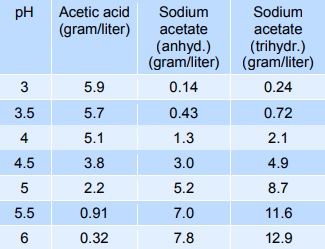 Source: chemicalbook.com
Source: chemicalbook.com
Ethyl acetate is the ester of ethanol. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each. 8829 C 1621 F specific gravity. Sodium Acetate Manufacturer Information Company Name. When the solution cools to room temperature it should solidify.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
1577 K Solubility in water. Ethyl ethanoate ethyl acetate is an ester. 56 C 1328 F Boiling point of alcohol. Upon contact with strong or weak acids Na 2 SO 3. Winchester of yellow paraffin oil.
The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Boiling point of water. Under standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions. When all Ethyl Acetate is distilled the temperature suddenly rises. Anhydrous calcium chloride lumps 50g dry in oven overnight.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Under standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions. Calcium chloride hydrate 6H2O 10g solid per pair. You can also heat the solution in the microwave for 1-2 minutes until boiling. Cayman Chemical Company Emergency. The general method of ester preparation can be.
Source:
Slowly pour the solution on top of the crystal. -1958 C -3204 F Boiling point of liquid helium. Sodium sulfite does not have a specific boiling point since it tends to decompose at high temperatures. An example of a supercooled liquid can be made by heating solid sodium acetate trihydrate NaCH 3 CO 2 3 H 2 O. 0971 20 C oxidation states 1 1 rare electron configuration.
Source: chemspider.com
Add one or two of the sodium acetate crystals thats right it only takes a single crystal to the center of a plate. -269 C -452 F. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C. At some point the Ethyl Acetate is going to get all distilled over. Reaction is given below Reaction with Sodium Hydroxide When ethyl acetate reacts with sodium.

Ethyl acetate is the ester of ethanol. It is moderately soluble in water its solubility corresponds to 27g100mL. This is because water is going to start to be distilled over 100ºC. If a small crystal of sodium acetate trihydrate is added to the liquid however. Ethylene vinyl acetate is a type of plastic a polymer with a wide variety of uses both residential and industrial.
 Source: researchgate.net
Source: researchgate.net
Slowly pour the solution on top of the crystal. Product and Company Identification Product Code. Saturated sodium carbonate solution 1 Bottle. 3732 K Boiling point of ethanol. Sodium Acetate MATERIAL SAFETY DATA SHEET 05201998 04282005 07122004 Date Created.
 Source: researchgate.net
Source: researchgate.net
It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid. A sharp boiling point is an indication of the purity of the ester. 2-8-1 or 1s 2 2s 2 2p 6 3s 1. The general method of ester preparation can be. It is widely used across a number of industrial sectors.
 Source: researchgate.net
Source: researchgate.net
7837 C 1731 F Boiling point of methanol. Sodium Acetate Manufacturer Information Company Name. It is widely used across a number of industrial sectors. An example of a supercooled liquid can be made by heating solid sodium acetate trihydrate NaCH 3 CO 2 3 H 2 O. Under standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of sodium acetate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.