Boiling point of phosphoric acid
Home » datasheet » Boiling point of phosphoric acidBoiling point of phosphoric acid
Boiling Point Of Phosphoric Acid. High pressure compressor oil 150 o C. Gear oil DTE extra heavy 150 o C. It is a weak acid as it does not fully dissociate in water. H 2 SO 4 Uses Sulfuric Acid It is used in making fertilizers.
 Phosphoric Acid Data Ml2r Consultancy From ml2rconsultancy.com
Phosphoric Acid Data Ml2r Consultancy From ml2rconsultancy.com
Molecular mass 97994 gmol boiling point 158 C and melting point 4235 C. Substance Substance name. When the boiling is over. The food industry continues to include phosphate additives in foods that would actually be low-phosphorus foods if left alone. It is used to produce phosphoric acid. Phosphoric Acid 85 ww Chemical name.
Phosphoric acid boiling point 3164F 158C Phosphoric acid charge 0.
UOP555-10 Trace Impurities in Benzene by GC. Arsenic acid as such has not been isolated but is. Di-2-ethylhexylphosphoric acid DEHPA or HDEHP is an organophosphorus compound with the formula C 8 H 17 O 2 PO 2 H. List two wide applications of phosphoric acid. H 2 SO 4 Uses Sulfuric Acid It is used in making fertilizers. What is the molecular mass boiling point and melting point of phosphoric acid.
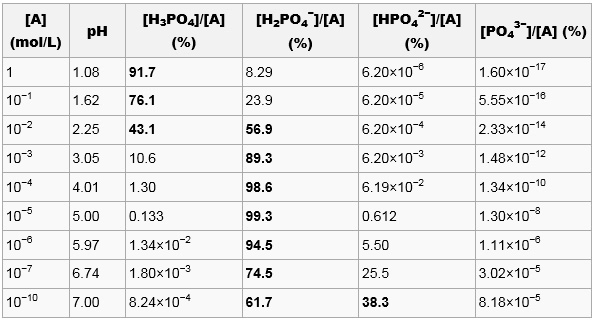 Source: hydro-land.com
Source: hydro-land.com
Absorbs moisture or water from the air. What is the molecular mass boiling point and melting point of phosphoric acid. Dehydrating agentcatalyst Type of reaction. UOP551-08 C 6 and Lower Boiling Hydrocarbons in Olefin Free Naphthas by GC. Hydrofluor acid 5 20 o C.
 Source: sciencedirect.com
Source: sciencedirect.com
UOP578-11 Automated Pore Volume and Pore Size Distribution of Porous Substances by Mercury Porosimetry. Hydrofluor acid 5 20 o C. It is used in the solvent extraction of uranium as well as the rare-earth metals. Acid catalysed elimination H2O C C H H H C H H H propan-1-ol propene Some 2o and 3o alcohols can give more than one product when the double bond forms between different carbon atoms C H C H C H H H. Gear oil DTE BB 150 o C.
 Source: bssa.org.uk
Source: bssa.org.uk
13 12212016 EN English US Page 1 SECTION 1. Gear oil DTE HH 150 o C. UOP578-11 Automated Pore Volume and Pore Size Distribution of Porous Substances by Mercury Porosimetry. Why is phosphoric acid in Coke and other consumable products. Gear oil DTE extra heavy 150 o C.
 Source: ml2rconsultancy.com
Source: ml2rconsultancy.com
Molecular mass 97994 gmol boiling point 158 C and melting point 4235 C. Glycerin 100 o C. It is used in the solvent extraction of uranium as well as the rare-earth metals. What is the molecular mass boiling point and melting point of phosphoric acid. UOP565-05 Acid Number and Naphthenic Acids by Titration.
 Source: sciencedirect.com
Source: sciencedirect.com
The colorless liquid is a diester of phosphoric acid and 2-ethylhexanol. It used as a cleaning agent in industries to remove the rust from steel and. Molecular mass 97994 gmol boiling point 158 C and melting point 4235 C. Glycerin 100 o C. UOP555-10 Trace Impurities in Benzene by GC.
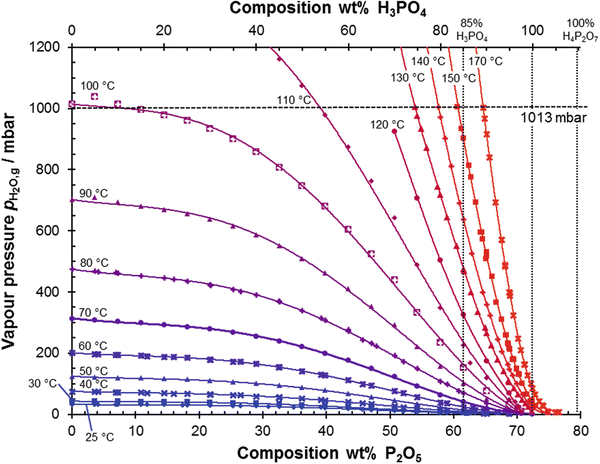 Source: link.springer.com
Source: link.springer.com
At this point the samples should. Warm under reflux Role of reagent. Gear oil DTE HH 150 o C. Specific GravityDensity1680 Molecular FormulaH3O4P Molecular Weight98 Section 10 - Stability and Reactivity Chemical Stability. Absorbs moisture or water from the air.
 Source: sciencedirect.com
Source: sciencedirect.com
UOP588-12 Total Inorganic and Organic. UOP565-05 Acid Number and Naphthenic Acids by Titration. It is a weak acid as it does not fully dissociate in water. Substance Substance name. Phosphoric acid is widely used in fertilizers.
Source: chemspider.com
Acid catalysed elimination H2O C C H H H C H H H propan-1-ol propene Some 2o and 3o alcohols can give more than one product when the double bond forms between different carbon atoms C H C H C H H H. Specific GravityDensity1680 Molecular FormulaH3O4P Molecular Weight98 Section 10 - Stability and Reactivity Chemical Stability. H 2 SO 4 Uses Sulfuric Acid It is used in making fertilizers. Sulfuric Acid Structure H 2 SO 4. List two wide applications of phosphoric acid.
Di-2-ethylhexylphosphoric acid DEHPA or HDEHP is an organophosphorus compound with the formula C 8 H 17 O 2 PO 2 H. Concentrated sulfuric or phosphoric acids Conditions. Phosphoric acid is widely used in fertilizers. Phosphoric Acid 85 ww Safety Data Sheet according to Federal Register Vol. Food and beverage manufacturers like it because its cheap adds tartness and acts as a preservative.
 Source: sciencedirect.com
Source: sciencedirect.com
Gear oil Type SEA 90 150 o C. Phosphoric Acid 85 ww Safety Data Sheet according to Federal Register Vol. Di-2-ethylhexylphosphoric acid DEHPA or HDEHP is an organophosphorus compound with the formula C 8 H 17 O 2 PO 2 H. UOP578-11 Automated Pore Volume and Pore Size Distribution of Porous Substances by Mercury Porosimetry. The food industry continues to include phosphate additives in foods that would actually be low-phosphorus foods if left alone.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of phosphoric acid by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.