Boiling point of pentanol
Home » datasheet » Boiling point of pentanolBoiling point of pentanol
Boiling Point Of Pentanol. 1312 22-dimethyl-1-propanol or neopentyl alcohol primary 22-Dimethylpropan-1-ol. Based on the type or types of intermolecular forces predict the substance in each pair that has the higher boiling point. 5000 mm Hg Congealing Point-1500 C. Which statement or statements about the boiling point of alkanes are true.
 3 Trends That Affect Boiling Points Chemistry By Mukesh Sharma From chemistrybymukesh.blogspot.com
3 Trends That Affect Boiling Points Chemistry By Mukesh Sharma From chemistrybymukesh.blogspot.com
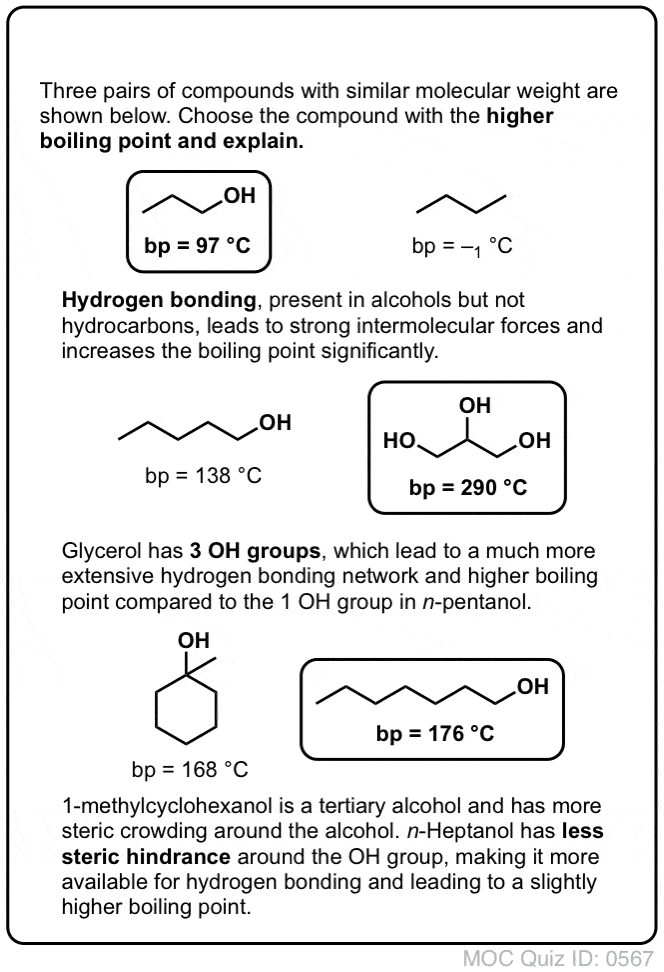
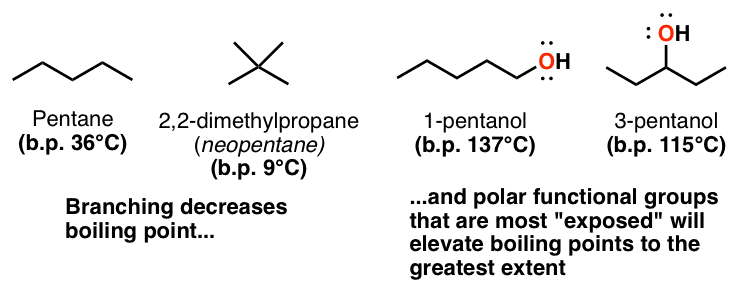
The working range is 15 to 150 mgcu m for a 10 liter air sample. The hydroxyl group of 1-pentanol is more exposed than it is in 3-pentanol which is flanked by two bulky alkyl groups so it will be better able to hydrogen bond with its fellows. Chapter 6 Amines and Amides 23 Some Important Alkaloids 24 Important Alkaloids N N N N O CH3 O CH3 CH3 Caffeine Found in the seeds of Coffea arabica roasted coffee beans. Ortho nitrophenol has lower boiling point than p-nitrophenol. Which statement or statements about the boiling point of alkanes are true. 0094000 mmHg 2500 C.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
Highest viscosity surface tension and boiling point pentanol pentanal pentane. Properties of Organic Solvents. It can also apply to hydrogen bonding molecules like alcohols compare the boiling points of 1-pentanol to 2-pentanol and 3-pentanol for instance. For Methyl isobutyl carbinol this method has an estimated detection limit of 001 mg for a 10 liter sample. Vapors heavier than air. 37 Air 1 Flash Point.
 Source: researchgate.net
Source: researchgate.net
Give a chemical test to distinguish between 2-Pentanol and 3-Pentanol. Pentane boils at a higher temperature than butane. Any potential health risk in the final material or article arising from their use should be assessed by the manufacturer in accordance with internationally recognised scientific principles on risk assessment. Hints for logging in with Active Directory. Of Iodoform while 3-pentanol does not give this test.

1385 2-methyl-1-butanol or active amyl alcohol primary 2-Methylbutan-1-ol. Hexane boils at a higher temperature than heptane. Write the chemical reaction to explain Kolbes reaction. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. The flexibility to have completely different styles of pages is just superb.
 Source: numerade.com
Source: numerade.com
The hydroxyl group of 1-pentanol is more exposed than it is in 3-pentanol which is flanked by two bulky alkyl groups so it will be better able to hydrogen bond with its fellows. Set up the distillation equipment as in the diagram on the right. Of Iodoform while 3-pentanol does not give this test. A sharp boiling point is an indication of the purity of the ester. 1200 months or longer if stored properly.

Vapors may irritate skin and eyes. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Hints for logging in with Active Directory. The working range is 15 to 150 mgcu m for a 10 liter air sample. For Methyl isobutyl carbinol this method has an estimated detection limit of 001 mg for a 10 liter sample.
 Source: chemistrybymukesh.blogspot.com
Source: chemistrybymukesh.blogspot.com
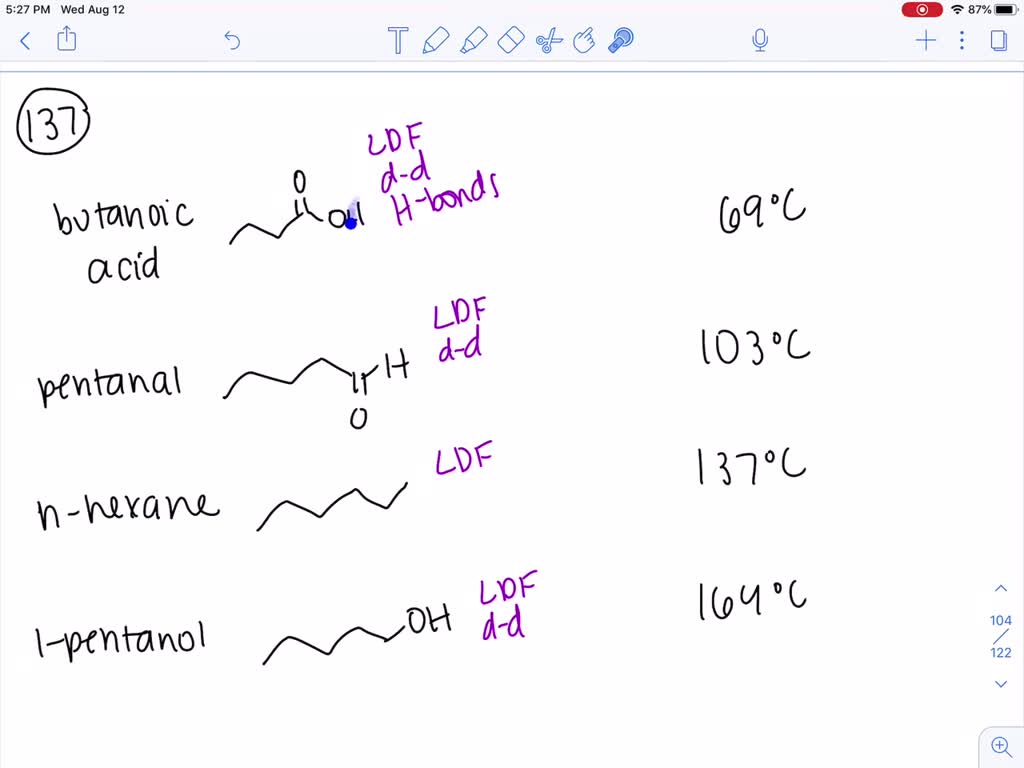
1-Pentanol or n-pentanol pentan-1-ol is an alcohol with five carbon atoms and the molecular formula C 5 H 11 OH. Used as a solvent and to make other chemicals. The working range is 15 to 150 mgcu m for a 10 liter air sample. All of the compounds have about the same molecular weight propanoic acid diethylamine 1-butanol. Given the molecules diethyl ether CH3CH2OCH2CH3 and 1-butanol CH3CH2CH2CH2OH 1-butanol CH3CH2CH2CH2OH has the higher boiling point mainly due to hydrogen bonding influences.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
To the mixture in the round bottom flask as prepared above add boiling chips to prevent bumping. For Methyl isobutyl carbinol this method has an estimated detection limit of 001 mg for a 10 liter sample. 1-Pentanol or n-pentanol pentan-1-ol is an alcohol with five carbon atoms and the molecular formula C 5 H 11 OH. Store in cool dry place in tightly sealed containers protected from heat and light. This method may be used to determine two or more.

Based on the type or types of intermolecular forces predict the substance in each pair that has the higher boiling point. Ortho nitrophenol has lower boiling point than p-nitrophenol. 12400 to 12500 C. All of the compounds have about the same molecular weight propanoic acid diethylamine 1-butanol. Boiling point C 1-pentanol or normal amyl alcohol primary Pentan-1-ol.
 Source: researchgate.net
Source: researchgate.net
I the mixture in the round bottom flask is best heated using a. Gas Chromatography Flame Ionization Detection. 12400 to 12500 C. To the mixture in the round bottom flask as prepared above add boiling chips to prevent bumping. Vapors may irritate skin and eyes.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Of Iodoform while 3-pentanol does not give this test. 1131 2-pentanol or sec-amyl alcohol or methyl n propyl carbinol. 2-pentanol gives Iodoform test with yellow ppt. It can also apply to hydrogen bonding molecules like alcohols compare the boiling points of 1-pentanol to 2-pentanol and 3-pentanol for instance. Less dense than water.
 Source: researchgate.net
Source: researchgate.net
Used as a solvent and to make other chemicals. I the mixture in the round bottom flask is best heated using a. 1131 2-pentanol or sec-amyl alcohol or methyl n propyl carbinol. This method may be used to determine two or more. Therefore they should at this point of time not be subject to the authorisation procedure at EU level.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of pentanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.