Boiling point of octane
Home » datasheet » Boiling point of octaneBoiling point of octane
Boiling Point Of Octane. If we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen. 56 C 1328 F Boiling point of alcohol. The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bond. The yield of gasoline may be doubled by converting higher or lower boiling point fractions into hydrocarbons in the gasoline range.
 Effect Of Blending Bioethanol On Boiling Point Of Octane Download Scientific Diagram From researchgate.net
Effect Of Blending Bioethanol On Boiling Point Of Octane Download Scientific Diagram From researchgate.net
Boiling point butane-05 1-butene-62 1-butyne 80 5 Melting point pentane-130 1-pentene-1652 1-pentyne-900 Boiling point pentane 36 1-pentene 299 1-pentyne 401 Just like their saturated counterparts the unsaturated hydrocarbons are usually non-polar. Boiling point of water. Kevin Hart John Krasinski Kate McKinnon Vanessa Bayer and Natasha Lyonne also lend their lungs. Propane Boiling Point - Water boils at 212F meaning that it becomes a gas at this temperature whereas water is still a liquid at 200F. Brake Parts Cleaner Lucas Non-Chlorinated Brake Parts Cleaner is manufactured with the highest quality components to provide excellent performance without leaving any residue. Use toluene in place of carbon disulfide for low-boiling analytes 5.
647 C 1485 F Boiling point of acetone.
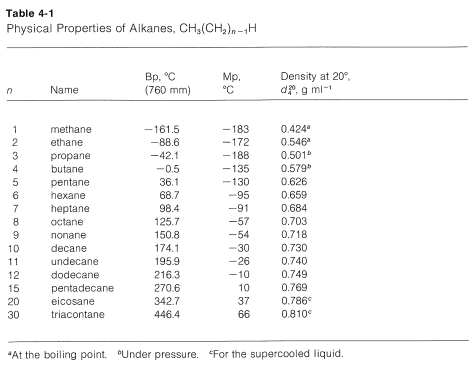
The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. Delayed Coking Unit DCU In the DCU the heavy residuum from the Crude Unit is poured into a large drum where it is heated to break down or crack it. Two of the. For compounds with the same carbon number the order of increasing boiling point by class is isoparaffin n-paraffin naphthene and aromatic.
 Source: thermopedia.com
Source: thermopedia.com
Compound Hydrocarbon Class Formula Boiling. Calculate the molar mass of the biomolecule. Compound Hydrocarbon Class Formula Boiling. Bicyclo222octane C8H14 CID 9235 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Two of the.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
The last compound an isomer of octane is nearly spherical and has an exceptionally high melting point only 6º below the boiling point. December 3 2016 at 335 pm. Here the crude is heated in a vacuum which lowers the boiling point of the fractions. Introduction to the Study of Chemistry - Atoms Elements Compounds Chemical Properties Physical Properties. The most powerful intermolecular force influencing neutral uncharged molecules is the hydrogen bond.
 Source: researchgate.net
Source: researchgate.net
101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. December 3 2016 at 335 pm. 56 C 1328 F Boiling point of alcohol. Hydrogen bonding is a special type of chemical bond that involves dipole-dipole attraction between two or more dipolar molecules which are also referred to simply as dipoles. Δt i K b m 135 C 1 503 C kg mol 1 x 00160 kg 135 C 314375 C mol.
 Source: oneclass.com
Source: oneclass.com
Boiling Point director and writer Philip Barantini. Calculate the molar mass of the biomolecule. A mixture of different compounds boils over a certain range of temperature reflecting the boiling point of each specific compound present in the mixture. 7837 C 1731 F Boiling point of methanol. Solid BOILING POINTS AND STRUCTURES OF HYDROCARBONS.
 Source: chem.libretexts.org
Source: chem.libretexts.org
C 12 H 26-10. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. 56 C 1328 F Boiling point of alcohol. If we compare the boiling points of methane CH 4 -161ºC ammonia NH 3 -33ºC water H 2 O 100ºC and hydrogen. Fuel Boiling Point o F Acetaldehyde.
 Source: butane.chem.uiuc.edu
Source: butane.chem.uiuc.edu
Delayed Coking Unit DCU In the DCU the heavy residuum from the Crude Unit is poured into a large drum where it is heated to break down or crack it. Melting freezing temperatures also increase with molecular weight but they are strongly influenced by the molecular shape. Dwayne Johnson is Krypto in DC League of Superpets. C 11 H 24-25. C 12 H 26-10.
 Source: researchgate.net
Source: researchgate.net
-1958 C -3204 F Boiling point of liquid helium. In other words at 10 degrees below zero propane is well past its boiling point. Delayed Coking Unit DCU In the DCU the heavy residuum from the Crude Unit is poured into a large drum where it is heated to break down or crack it. This means the intermolecular forces between unsaturated hydrocarbon molecules are dominantly weak Van der Waals force. This fractional distillation process yields approximately 250 mL of straight-run gasoline for each liter of crude oil.
 Source: youtube.com
Source: youtube.com
Solid BOILING POINTS AND STRUCTURES OF HYDROCARBONS. In other words at 10 degrees below zero propane is well past its boiling point. Some fuels and their boiling points at atmospheric pressure. Petrol is produced first in this process as it is produced at a lower temperature than diesel. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. C 20 H 42. How chlorine will affect the boiling point of a alkyl chain for exemple 1-chloro-octane compare to octane. Petrol is produced first in this process as it is produced at a lower temperature than diesel. -1958 C -3204 F Boiling point of liquid helium.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When used as a test fuel component in anti-knock test engines a 100 heptane fuel is the zero point of the octane rating scale the 100 point is 100 iso-octaneOctane number equates to the anti-knock qualities of a comparison. C 8 H 18-57. Read More 5k. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. For many purposes it is suitably to calculate an average boiling point ABP of mixtures.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of octane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.