Boiling point of nonane
Home » datasheet » Boiling point of nonaneBoiling point of nonane
Boiling Point Of Nonane. C 10 H 22-30. Air - Molecular Weight and Composition - Dry air is a. Hexadecane Cetane CH 3 CH 2 14 CH 3 C 16 H 34 3D. The midweight alkanes are liquids.
 Hydrocarbons Physical Data From engineeringtoolbox.com
Hydrocarbons Physical Data From engineeringtoolbox.com
A liquid boils when its vapor. And the heavier alkanes are solids or tars. B Nonane is a gas at room temperature. Propane crystallizes in the space group P2 1 n. Which of the following is true of nonane C₉H₂₀ which has a density of 079 gmL melts at -51 C and boils at 151 C. 101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure.
There is no simple arithmetic relationship between the number of carbon atoms in a formula and the number of isomers.
Yet the boiling point of n-nonane which has one less ceCH2 group ceC9H20 isomer is pu1508 mathrmoC. Hexadecane is typical for the number of carbons in the hydrocarbons present in diesel fuel. Boiling Point 8160 C at 1 atm. The low spacefilling of 585 at 90 K due to the bad stacking properties of the molecule is the reason for the particularly low melting point. Melting Point o C Boiling Point o C State at 25 o C. Yaws CL Chemical Properties Handbook.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Nonane has a boiling point of 1508C. The boiling points of organic compounds can give important clues to other physical properties and structural characteristics. For example n-butane has a higher boiling point 05 C 311 F than isobutane 117 C 109 F. Propane is a colorless odorless gas. Decane CH 3 CH 2 8 CH 3 C 10 H 22 3D.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
There are 75 structural isomers of C 10 H 22. There are 75 structural isomers of C 10 H 22. 101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. C 11 H 24-25. At normal pressure it liquifies below its boiling point at 42 C and solidifies below its melting point at 1877 C.
Source: quora.com
And the heavier alkanes are solids or tars. This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. Hexadecane is typical for the number of carbons in the hydrocarbons present in diesel fuel. And the heavier alkanes are solids or tars. E Nonane floats on.
 Source: en.wikipedia.org
Source: en.wikipedia.org
C 30 H 62. 32 grams of a compound X when dissolved in 450 grams of. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. D Nonane does not undergo combustion. C 9 H 20-51.
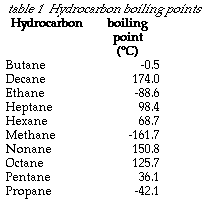 Source: quotesgram.com
Source: quotesgram.com
Boiling Point 8160 C at 1 atm. C 10 H 22-30. And the heavier alkanes are solids or tars. C 12 H 26-10. At room temperature the lighter alkanes are gases.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Boiling Point 8160 C at 1 atm. C 30 H 62. C 2 H 6 1833 886. Properties such as melting point and boiling point usually change smoothly and predictably as the number of carbon and hydrogen atoms in the molecules change. Coefficents calculated by NIST from authors data.
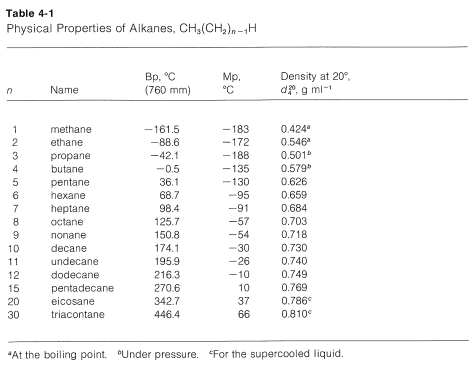 Source: chem.libretexts.org
Source: chem.libretexts.org
C 2 H 6 1833 886. Decane CH 3 CH 2 8 CH 3 C 10 H 22 3D. 1 Melting Point -457 C 2 Density 077674 gcm 3 at 25C 3 Vapor Density 143 relative air1 Coefficient of Thermal Expansion 0001397 C-1 4 Refractive Index 13442 Na D at 20C 5 Viscosity 0352 cP at 20C 6 Triple Point -438 C 7 Critical Temperature 2724 C 8 Critical Pressure 483 MPa 8 Critical Volume 0173 L Surface Tension 2929 dynecm at. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. Which of the following is true of nonane C₉H₂₀ which has a density of 079 gmL melts at -51 C and boils at 151 C.
 Source: chegg.com
Source: chegg.com
Properties such as melting point and boiling point usually change smoothly and predictably as the number of carbon and hydrogen atoms in the molecules change. The low spacefilling of 585 at 90 K due to the bad stacking properties of the molecule is the reason for the particularly low melting point. CH 4-1825 -164. Air - Molecular Weight and Composition - Dry air is a. Propane crystallizes in the space group P2 1 n.
 Source: youtube.com
Source: youtube.com
The boiling points of organic compounds can give important clues to other physical properties and structural characteristics. The midweight alkanes are liquids. Some fluids and their dielectric constants or permittivities. C Nonane is a solid at room temperature. The number of.

CH 4-1825 -164. If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. Physical Thermo-dynamic Environmental. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. Decane CH 3 CH 2 8 CH 3 C 10 H 22 3D.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of nonane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.