Boiling point of n butanol
Home » datasheet » Boiling point of n butanolBoiling point of n butanol
Boiling Point Of N Butanol. N-butanol 2-butanol 23-butanediol 3-hydroxy-2-butanone and 23-butanedione. 562 167 948 K b. 2989 44 39 Acetic acid. Energy Forms.
 3 Trends That Affect Boiling Points Master Organic Chemistry From masterorganicchemistry.com
3 Trends That Affect Boiling Points Master Organic Chemistry From masterorganicchemistry.com
B Linear n-pentane. The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. A In this series of four simple alkanes larger molecules have stronger London forces between them than smaller molecules and consequently higher boiling points. 562 167 948 K b. 462 234 1115. N-butanol 2-butanol 23-butanediol 3-hydroxy-2-butanone and 23-butanedione.
Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source.
Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source. Butan-1-ol also known as n-butanol is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. 801 265 55 512 K b K f. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
As a result the boiling point of neopentane 95C is more than 25C lower than the boiling point of n-pentane 361C. Samples from three individuals who died following LPG abuse contained a range of putative n-butane metabolites. The two sets of coefficients give different results at the NBP temperature. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and. Mass and Surface Area Affect the Strength of London Dispersion Forces.
 Source: thermopedia.com
Source: thermopedia.com
562 167 948 K b. Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer. The two sets of coefficients give different results at the NBP temperature. N-butanol 2-butanol 23-butanediol 3-hydroxy-2-butanone and 23-butanedione. B Linear n-pentane.
 Source: iea-amf.org
Source: iea-amf.org
Direction of Heat. 2989 44 39 Acetic acid. 1179 307 166 390 K b K f. 1560 626 306 Camphor. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol.

Energy Forms. 2989 44 39 Acetic acid. The greatly increased boiling point is due to the fact that butanol contains hydroxyl group which is capable of hydrogen bonding. The hydrolysis of n-butyl acetate in blood and brain is estimated to be 99 complete by 27 min at this. N-butanol 2-butanol 23-butanediol 3-hydroxy-2-butanone and 23-butanedione.
 Source: iea-amf.org
Source: iea-amf.org
To the authors knowledge the last three compounds have not been proposed as metabolites of n-butane in man. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. Academiaedu is a platform for academics to share research papers. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water.
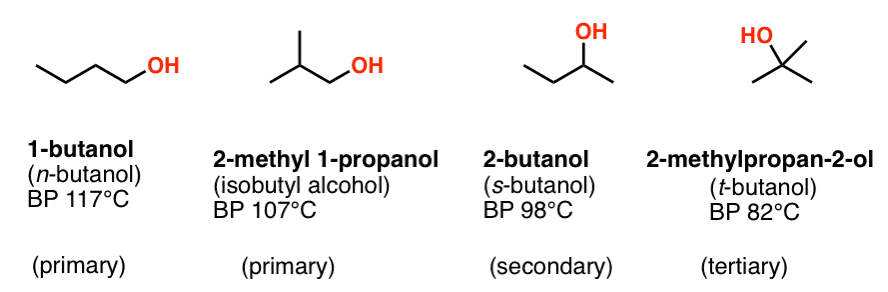 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
1179 307 166 390 K b K f. Samples from three individuals who died following LPG abuse contained a range of putative n-butane metabolites. Academiaedu is a platform for academics to share research papers. The boiling point of n-butanol is 117 o C. Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer. 1560 626 306 Camphor. 2040 595 179 40 K f. 1179 307 166 390 K b K f. Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source.
 Source: lobachemie.com
Source: lobachemie.com
The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer. The boiling point of n-butanol is 117 o C. B Linear n-pentane. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and other saccharides and is present in many foods and.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Isomers of butan-1-ol are isobutanol butan-2-ol and tert-butanolThe unmodified term butanol usually refers to the straight chain isomer. Samples from three individuals who died following LPG abuse contained a range of putative n-butane metabolites. 801 265 55 512 K b K f. Butan-1-ol also known as n-butanol is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. 1179 307 166 390 K b K f.
Butan-1-ol also known as n-butanol is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. B Linear n-pentane. These might be produced through similar metabolic pathways to those of. 23 K to 353. 2989 44 39 Acetic acid.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of n butanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.