Boiling point of mgo
Home » datasheet » Boiling point of mgoBoiling point of mgo
Boiling Point Of Mgo. Soluble in acid insoluble in base reacts with ethanol. On the other hand prolonged skin exposure to this oil can cause. The EJ201 and EJ202 engines had multi-point sequential fuel injection and centrally located spark plugs. The POM class is very similar to PAH compounds.
 Magnesium Oxide Wikipedia From en.wikipedia.org
Magnesium Oxide Wikipedia From en.wikipedia.org
First mark for S in box 1 AND R in box 3. Magnesium hydroxide forms in the presence of water. For example elemental sulfur is a yellow crystalline solid that does not conduct electricity and has a melting point of 1152 C no matter what amount is examined Figure PageIndex1. Thus beyond the critical point we refer to this single phase as a supercritical fluid. Teat pipettedropping pipette. The ignition knock control system had fuzzy logic that enabled the maximum ignition advanced angle to be used without detonation.
M2 - E 1.
Chlorine exists as a diatomic molecule. And boil The End Chemical Bond A bond results from the attraction of nuclei for electrons All atoms trying to achieve a stable octet IN OTHER WORDS the p in one nucleus are attracted to the e. Lactobacillus bacterium helps in the formation of _____. Hexane C 6H 14 mw86 has a boiling point of 68º. Find the latest Las Vegas Raiders news rumors trades free agency updates and more from the insider fans and analysts at Just Blog Baby. E - pipette.
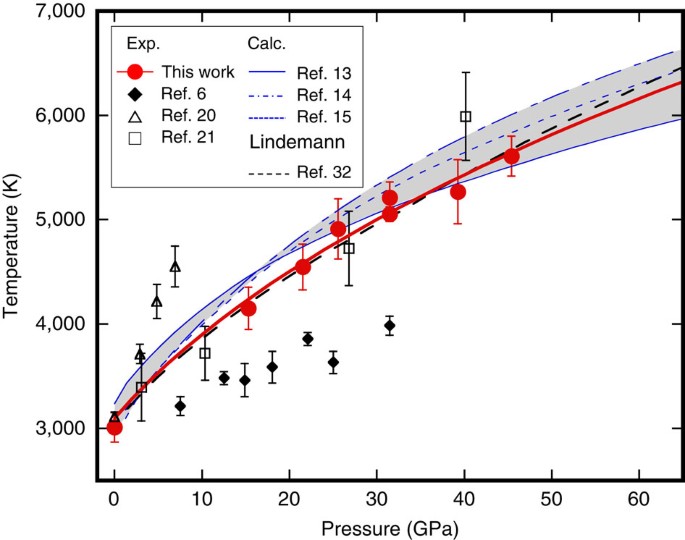 Source: nature.com
Source: nature.com
The freezing point is ____0C at which water freezes. The EJ201 and EJ202 engines had two ignition coils one for each pair of cylinders ie. Ionic bonds between Mg 2 and O 2-ions are stronger than those between Na and Cl-ions. It melts at an even lower temperature than the boiling point of that material. On the other hand prolonged skin exposure to this oil can cause.
 Source: chemistryworld.com
Source: chemistryworld.com
Which substance has the highest boiling point. This explains the higher stability of rmMgO compared to rmNaClrm Thus the Higher the magnitude of the charges of ions the higher is its lattice energy the more stable is the ionic compound 2 Let us consider rmNaCl and rmCsCl These two ionic compounds are univalent ie. Boiling point - the temperature at which a liquid turns into a gas. Soluble in acid insoluble in base reacts with ethanol. Find the latest Las Vegas Raiders news rumors trades free agency updates and more from the insider fans and analysts at Just Blog Baby.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
930 K decomposes Solubility in water. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. And boil The End Chemical Bond A bond results from the attraction of nuclei for electrons All atoms trying to achieve a stable octet IN OTHER WORDS the p in one nucleus are attracted to the e. Evidently with its extra mass it has much stronger London dispersion attraction enough so to overcome the dipole advantage of HCl. Magnesium hydroxide forms in the presence of water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Which substance has the highest boiling point. E - pipette. Intensive properties in contrast do not depend on the amount of the substance. 930 K decomposes Solubility in water. 1-2 and 3-4 which fired the spark plugs directly twice per cycle.
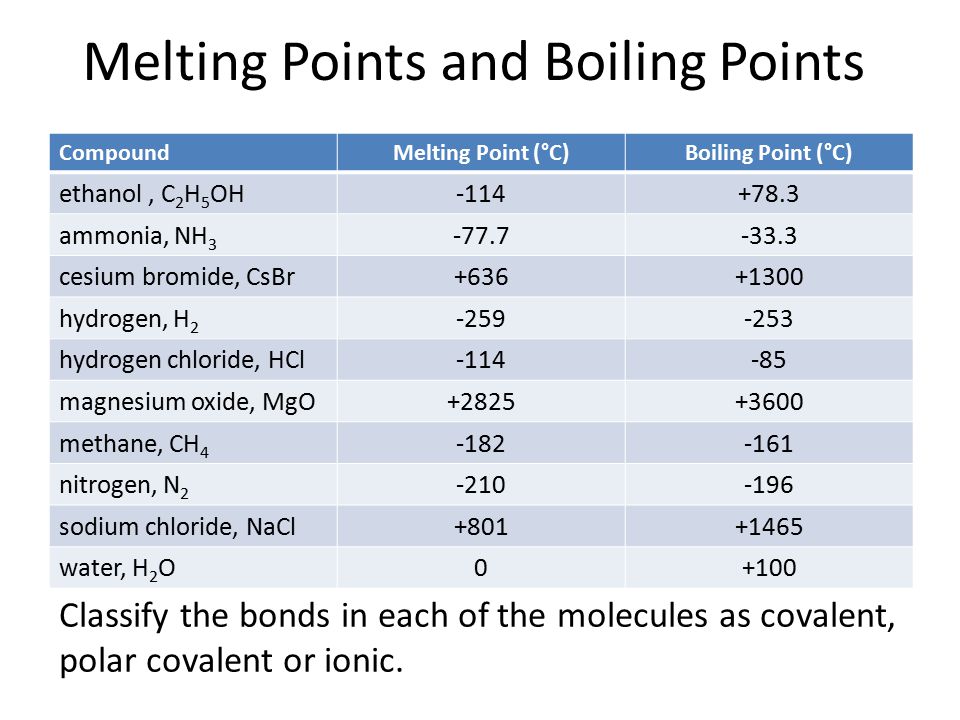 Source: slideplayer.com
Source: slideplayer.com
Boiling point - the temperature at which a liquid turns into a gas. Hexagonal Thermochemistry Heat capacity C 8937 JmolK Std molar entropy S o 298 95 JmolK Std enthalpy of formation Δ f H 298. A group of seven polynuclear aromatic hydrocarbons benzaanthracene benzobfluoranthene benzokfluoranthene benzoapyrene chrysene 712. 657 C 1215 F. Ethanol CH 3CH 2OH mw46 has a boiling point of 78º.
 Source: crystran.co.uk
Source: crystran.co.uk
1 Total marks for Question 1 6 marks Question number Answer Accept Reject Marks 2 a i D - hydrocarbons 1 b S U R V T. The EJ201 and EJ202 engines had two ignition coils one for each pair of cylinders ie. Schistosomiasis induces plasma cell death in the bone marrow and suppresses the efficacy of anti-viral vaccination The immunological role of cell wall components from diverse Mycobacterium tuberculosis clinical isolates - July 2021. The end of the boiling curve separating the liquid to vapor transition is called the critical point. Soluble in acid insoluble in base reacts with ethanol.
 Source: youtube.com
Source: youtube.com
Chlorine exists as a diatomic molecule. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Inhalation of paraffin oil can irritate the respiratory tract and cause cough shortness of breath and occasionally lead to hydrocarbon pneumonitis. The charges of the cations and anions are one. D test tubeboiling tube.
 Source: youtube.com
Source: youtube.com
Has a higher boiling point proves that it is has stronger intermolecular attractions despite its lesser dipole moment. Hexane C 6H 14 mw86 has a boiling point of 68º. The US EPA introduces the term polycyclic organic matter POM defined as a class of air toxic compounds with more than one benzene ring and a boiling point of 100C and higher. The end of the boiling curve separating the liquid to vapor transition is called the critical point. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils.
Source: quora.com
Galvanization is the process of coating iron objects with the layer of _____ metal. They include color melting point boiling point electrical conductivity and physical state at a given temperature. This explains the higher stability of rmMgO compared to rmNaClrm Thus the Higher the magnitude of the charges of ions the higher is its lattice energy the more stable is the ionic compound 2 Let us consider rmNaCl and rmCsCl These two ionic compounds are univalent ie. 1 b M1 - A. The end of the boiling curve separating the liquid to vapor transition is called the critical point.
 Source: crystran.co.uk
Source: crystran.co.uk
This type of glass melts easily at a relatively lower. 3 MgO MgCl 2 NaCl. Melting point - the temperature at which a solid turns into a liquid. Soluble in acid insoluble in base reacts with ethanol. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of mgo by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.