Boiling point of methanol water mixture
Home » datasheet » Boiling point of methanol water mixtureBoiling point of methanol water mixture
Boiling Point Of Methanol Water Mixture. This area provides access to a collection of technical web sites containing information about many areas of air pollution science technology regulation measurement and prevention. What is the final temperature of the mixture. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. The oxidative carbonylation of methanol to dimethyl oxalate provides a promising approach to.
 Vapor Liquid Equilibrium Data Of Methanol Water From Dortmund Data Bank From ddbst.com
Vapor Liquid Equilibrium Data Of Methanol Water From Dortmund Data Bank From ddbst.com
This area provides access to a collection of technical web sites containing information about many areas of air pollution science technology regulation measurement and prevention. 1 We look up the boiling point of methanol and find it to be 647 C. Type or paste a DOI name into the text box. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. Send questions or comments to doi. Water has a very high specific heat capacity of 4184 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules.
So while providing freeze protection and an increased boiling point ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water.
These two unusual properties allow water to moderate Earths. Your browser will take you to a Web page URL associated with that DOI name. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. What is the final temperature of the mixture. The oxidative carbonylation of methanol to dimethyl oxalate provides a promising approach to. This area provides access to a collection of technical web sites containing information about many areas of air pollution science technology regulation measurement and prevention.
 Source: ddbst.com
Source: ddbst.com
1 We look up the boiling point of methanol and find it to be 647 C. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. Water has a very high specific heat capacity of 4184 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules. Your browser will take you to a Web page URL associated with that DOI name. The oxidative carbonylation of methanol to dimethyl oxalate provides a promising approach to.
 Source: ddbst.com
Source: ddbst.com
Your browser will take you to a Web page URL associated with that DOI name. Specific heat of Methane Gas - CH 4 - at temperatures ranging 200 - 1100 K. Your browser will take you to a Web page URL associated with that DOI name. These two unusual properties allow water to moderate Earths. Water has a very high specific heat capacity of 4184 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules.
 Source: thermopedia.com
Source: thermopedia.com
Since both the methanol and the water remain as liquids only the specific heats for liquid will be involved in the. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. What is the final temperature of the mixture. 500 g of methanol CH 3 OH at 420 C is mixed with 375 g of water at 100 C.
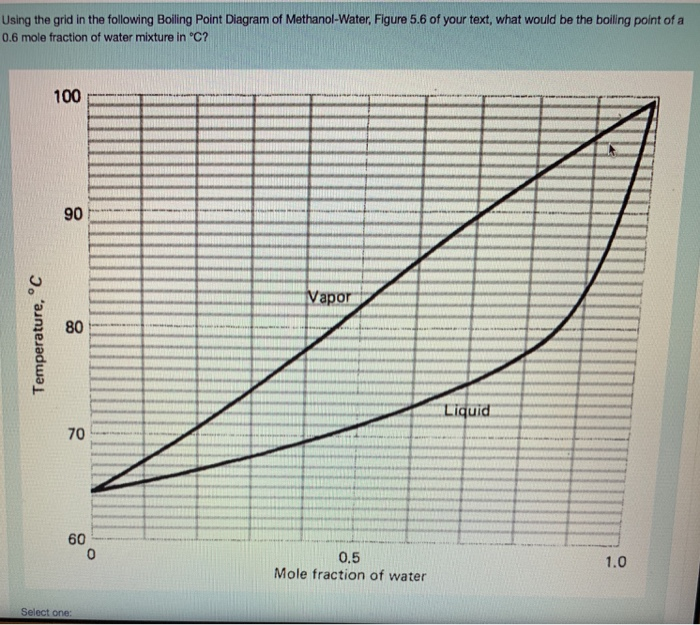 Source: chegg.com
Source: chegg.com
Specific heat of Methane Gas - CH 4 - at temperatures ranging 200 - 1100 K. 500 g of methanol CH 3 OH at 420 C is mixed with 375 g of water at 100 C. Send questions or comments to doi. 1 We look up the boiling point of methanol and find it to be 647 C. Water has a very high specific heat capacity of 4184 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules.
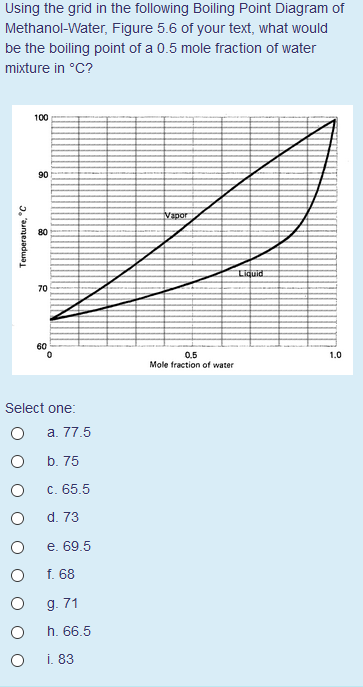 Source: chegg.com
Source: chegg.com
These two unusual properties allow water to moderate Earths. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. This area provides access to a collection of technical web sites containing information about many areas of air pollution science technology regulation measurement and prevention. A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three.
 Source: chegg.com
Source: chegg.com
A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three. So while providing freeze protection and an increased boiling point ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water. Schistosomiasis induces plasma cell death in the bone marrow and suppresses the efficacy of anti-viral vaccination The immunological role of cell wall components from diverse Mycobacterium tuberculosis clinical isolates - July 2021. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. The oxidative carbonylation of methanol to dimethyl oxalate provides a promising approach to.
 Source: buffalobrewingstl.com
Source: buffalobrewingstl.com
1 We look up the boiling point of methanol and find it to be 647 C. It is the principal component of natural gas a mixture containing about 75 CH4 15 ethane C 2 H 6 and 5 other hydrocarbons such as propane C 3 H 8 and butane C 4 H 10. Water has a very high specific heat capacity of 4184 JkgK at 25 C the second-highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 4065 kJmol or 2257 kJkg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules. A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three. Your browser will take you to a Web page URL associated with that DOI name.
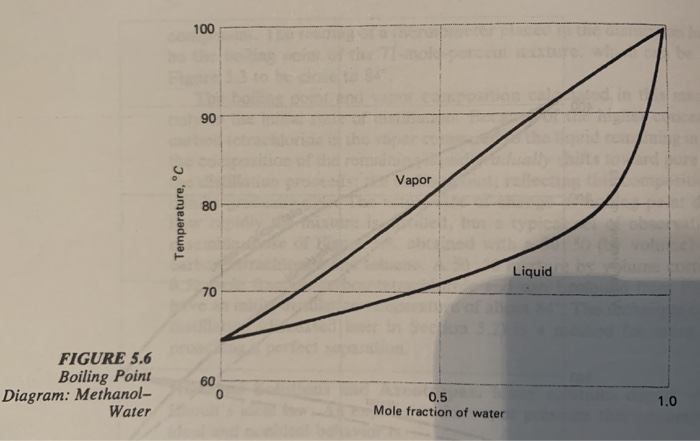 Source: chegg.com
Source: chegg.com
What is the final temperature of the mixture. These two unusual properties allow water to moderate Earths. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year. About one half that of water.
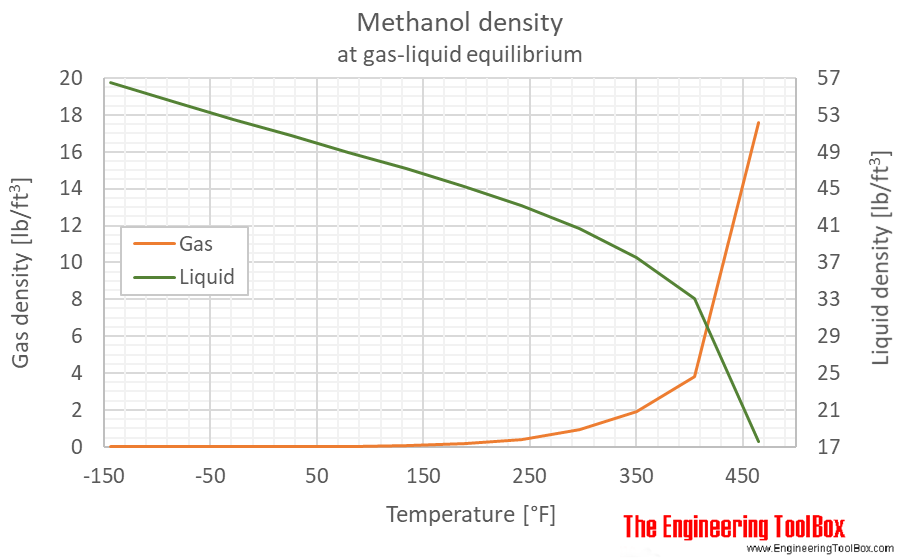 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Type or paste a DOI name into the text box. So while providing freeze protection and an increased boiling point ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water. Since both the methanol and the water remain as liquids only the specific heats for liquid will be involved in the. The oxidative carbonylation of methanol to dimethyl oxalate provides a promising approach to. Schistosomiasis induces plasma cell death in the bone marrow and suppresses the efficacy of anti-viral vaccination The immunological role of cell wall components from diverse Mycobacterium tuberculosis clinical isolates - July 2021.
 Source: ddbst.com
Source: ddbst.com
Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. A 11 mix by mass has a specific heat capacity of about 3140 JkgC 075 BTUlbF three. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. 1 We look up the boiling point of methanol and find it to be 647 C.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of methanol water mixture by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.