Boiling point of magnesium
Home » datasheet » Boiling point of magnesiumBoiling point of magnesium
Boiling Point Of Magnesium. Metallic lattice important to decide melting points of metals such as sodium magnesium and other metal elements Ionic lattice - In ionic compounds such as NaCl CaF 2 MgO ionic lattice exist. It is the temperature at which the solid phase changes to the liquid phaseThis is the point at which both liquid and solid phases exist at equilibriumVisit BYJUS to learn more about the Principle Detailed Explanation Videos and FAQs of melting point and Boiling point. Elemental magnesium is a gray-white lightweight metal and occurs naturally only in combination with other elements. Occurrence properties and uses.
 What Is The Melting Point Of Magnesium A Scientific Look At Magnesium Sophisticated Edge From sophisticatededge.com
What Is The Melting Point Of Magnesium A Scientific Look At Magnesium Sophisticated Edge From sophisticatededge.com
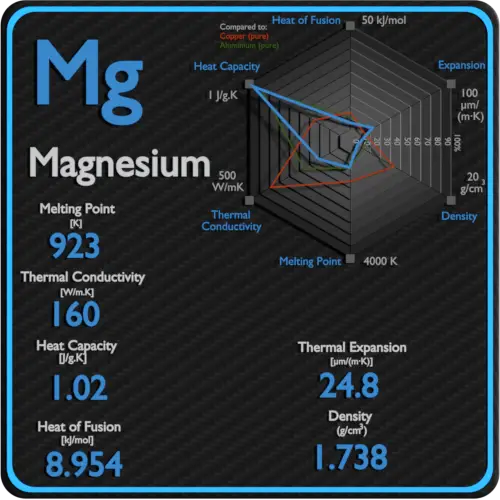
Melting Point and Boiling point- Melting point is a characteristic property of solid crystalline substances. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. Boiling point of Magnesium is 1090C. Hexagonal Density 293 K. If you come across an explanation for the very small increase in melting point from magnesium to aluminium in terms of the strength of the metallic bond you should be very wary of it unless it also explains why despite that the boiling point of aluminium is much higher than that of magnesium. Magnesium is used in super-strong lightweight.
1090 C 1994 F specific gravity.
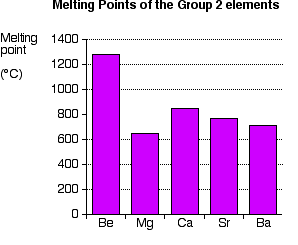
Boiling point - the temperature at which a liquid turns into a gas. In general boiling is a phase change of a substance from the liquid to the gas phase. Melting point - the temperature at which a solid turns into a liquid. Melting point of Magnesium is 649C. They have metallic bonding in which the nuclei of metal atoms are attracted to delocalised electrons. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure.
 Source: pinterest.com
Source: pinterest.com
They have metallic bonding in which the nuclei of metal atoms are attracted to delocalised electrons. The boiling point of aluminium is much higher than magnesiums - as you would expect. Note that these points are associated with the standard atmospheric pressure. 000008988 gas 273K 2. Magnesium is the eighth most abundant element in the Earths crust but does not occur uncombined in nature.
 Source: youtube.com
Source: youtube.com
The metal can be produced artificially but is highly reactive. Chemical properties such flammability and acidity and chemical changes such as rusting involve production of matter that differs from that present beforehand. Hexagonal Density 293 K. 000008988 gas 273K 2. A silylenoid is a compound that contains R 2 SiM X M is metal and R is an organic moiety.
 Source: material-properties.org
Source: material-properties.org
Measurable properties fall into one of two categories. 7837 C 1731 F Boiling point of methanol. For example water boils at 100C 212F at sea level but at 934C 2001. 7837 C 1731 F Boiling point of nitrogen. Sodium magnesium and aluminium.
Source: quora.com
1250 C 2280 F. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Melting point - the temperature at which a solid turns into a liquid. The melting points and boiling.
 Source: researchgate.net
Source: researchgate.net
Just like how the strength of the bonds between atoms affect the Melting Point the boiling point depends on the heat energy required to create a transition from liquid to gaseous state. Hexagonal Density 293 K. S Density g cm 3 174 Atomic number. 6500 C 92315 K 12020 F Boiling Point. Metallic lattice important to decide melting points of metals such as sodium magnesium and other metal elements Ionic lattice - In ionic compounds such as NaCl CaF 2 MgO ionic lattice exist.
 Source: onyxmet.com
Source: onyxmet.com
Occurrence properties and uses. The sea contains trillions of tonnes of magnesium and this is the source of much of. S Density g cm 3 174 Atomic number. 1s 2 2s 2 2p 6 3s 2. 000008988 gas 273K 2.
 Source: sophisticatededge.com
Source: sophisticatededge.com
In general boiling is a phase change of a substance from the liquid to the gas phase. Melting Point and Boiling point- Melting point is a characteristic property of solid crystalline substances. These salts are typical ionic halides being highly soluble in waterThe hydrated magnesium chloride can be extracted from brine or sea waterIn North America magnesium chloride is produced primarily from Great. 1090 C 1994 F specific gravity. Magnesium is used in super-strong lightweight.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Melting point of Magnesium is 649C. 12 Relative atomic mass. 1250 C 2280 F. Hexagonal Density 293 K. 100 C 212 F Boiling point of water in Kelvin.
 Source: researchgate.net
Source: researchgate.net
1520 K Solubility in water. Metals - Latent. 56 C 1328 F Boiling point of alcohol. Boiling point of Magnesium is 1090C. 1s 2 2s 2 2p 6 3s 2.
 Source: chemguide.co.uk
Source: chemguide.co.uk
2 - Atomic number-253. Boiling point of water. Click on any elements name for further chemical properties environmental data or health effects. 1090C 1994F 1363 K Block. In the dissolved state magnesium binds hydration water tighter than calcium potassium and sodium.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of magnesium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.