Boiling point of liquid propane
Home » datasheet » Boiling point of liquid propaneBoiling point of liquid propane
Boiling Point Of Liquid Propane. Propane exists in its liquid form at or below its boiling point -44F as well as when it stored under pressure. Hornback propane has a boiling point of -44 F -42 C at atmospheric pressure but methane natural gas has a boiling point of -260 F -162 C at atmospheric pressure. These are isomers having the same molecular formula C 5 H 12 but differ in their structures. Mark each of the following statements as TRUE or FALSE.
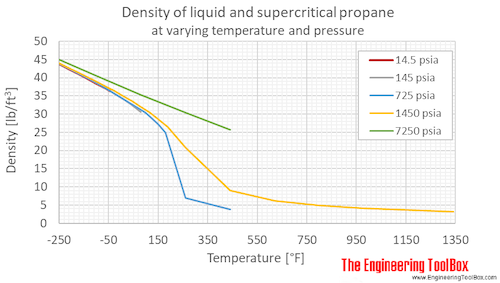 Propane Density And Specific Weight Vs Temperature And Pressure From engineeringtoolbox.com
Propane Density And Specific Weight Vs Temperature And Pressure From engineeringtoolbox.com
WarnerMedia uses data to improve and analyze its functionality and to tailor. According to the textbook Organic Chemistry by Joseph M. Because the boiling point of a liquid rises with pressure the contents of the pressurized vessel can remain liquid so long as the vessel is intact. In general blends with smaller. The boiling point of water is typically considered to be 100 C or 212 F. Both hexane and.
Propane stays liquid above the propane boiling point because it is under pressure in a gas cylinder.
14 mw86 has a boiling point of 68º. Propane stays liquid above the propane boiling point because it is under pressure in a gas cylinder. In polar compounds the positive. The same holds true for propane and is explained in detail below. 5 Related Records Expand this section. Ethanol CH 3CH 2OH mw46 has a boiling point of 78º.
 Source: elgas.com.au
Source: elgas.com.au
In polar compounds the positive. At the boiling point molecules anywhere in the liquid may be vaporized. See the table for biodiesels physical characteristics. Both hexane and. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F.
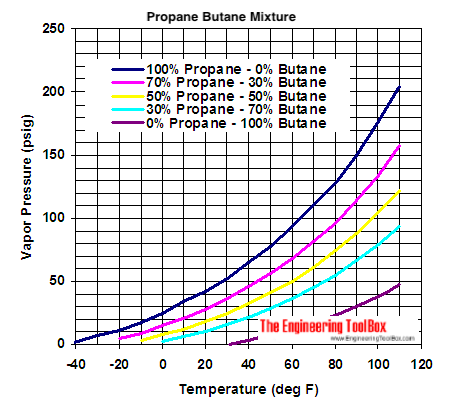 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Hornback propane has a boiling point of -44 F -42 C at atmospheric pressure but methane natural gas has a boiling point of -260 F -162 C at atmospheric pressure. The boiling point temperature will be lower if the atmospheric pressure is decreased. Per square inch absolute at 100 F. 5 Related Records Expand this section. Some fuels and their boiling points at atmospheric pressure.
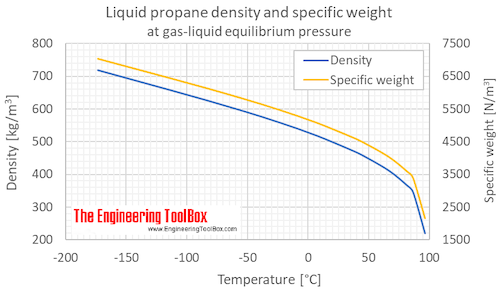 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
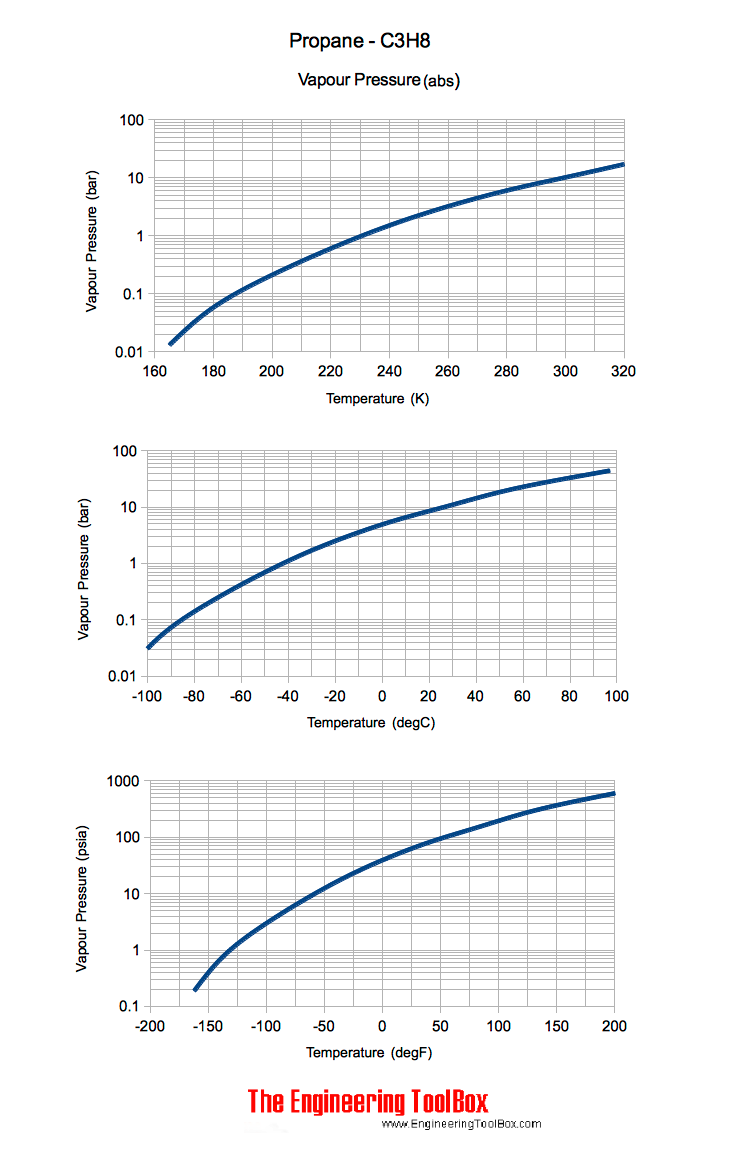
They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. All boiling points below are normalatmospheric boiling points. A flammable liquid is one having a flash point below 100 F 378 C and having a vapor pressure not exceeding 40 lbs.
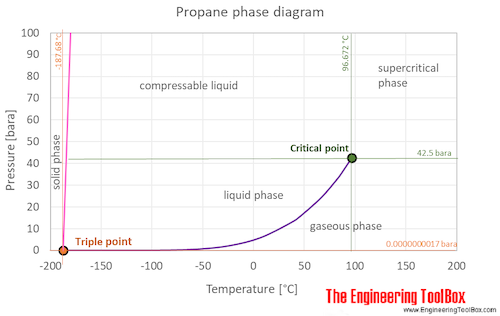 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
In contrast natural gas methane has a boiling point of -1615C -2587F at atmospheric pressure. Mark each of the following statements as TRUE or FALSE. WarnerMedia uses data to improve and analyze its functionality and to tailor. All boiling points below are normalatmospheric boiling points. Propane is the principal ingredient of bottled gas particularly in northern states whereas butane with its considerably higher boiling and freezing points is more widely used in warmer southern states.
 Source: elgas.com.au
Source: elgas.com.au
The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. Catch up with Stephen Colbert Henry Winkler and more of Anderson Coopers friends on his 24 hour streaming channel. Propane boiling point is -42C or -44F at atmospheric pressure the point at which liquid propane vaporises into gaseous propane. Propane exists in its liquid form at or below its boiling point -44F as well as when it stored under pressure. A boiling liquid expanding vapor explosion BLEVE ˈ b l ɛ v iː BLEV-ee is an explosion caused by the rupture of a vessel containing a pressurized liquid that has reached temperatures above its boiling point.
 Source: schoolscience.co.uk
Source: schoolscience.co.uk
Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure thus facilitating transition of the material between gaseous and liquid phases. Because the boiling point of a liquid rises with pressure the contents of the pressurized vessel can remain liquid so long as the vessel is intact. Propane stays liquid above the propane boiling point because it is under pressure in a gas cylinder. Polarity of the molecule determines the force of attraction between the molecules in the liquid state.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Product Boiling Point at Atmospheric. Boiling is the method of cooking food in boiling water or other water-based liquids such as stock or milk. WarnerMedia uses data to improve and analyze its functionality and to tailor. Per square inch absolute at 100 F. Combustibles are further separated into Category I.
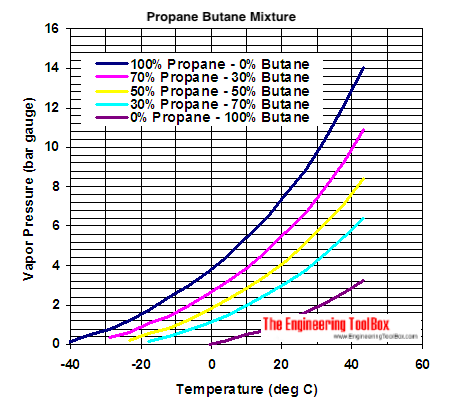 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Biodiesel performance in cold weather depends on the blend of biodiesel the feedstock and the petroleum diesel characteristics. 3 Chemical and Physical Properties Expand this section. A variety of alkanes with the generic formula C n H 2 n 2 are given in the table at the left with names formulas and physical properties. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. Some fuels and their boiling points at atmospheric pressure.
 Source: thermopedia.com
Source: thermopedia.com
An open tank would typically be used for cleaning at less than the boiling point cold cleaning. The same holds true for propane and is explained in detail below. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. Flammability solid gas Extremely flammable gas.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Melting pointfreezing point-3064 F -188 C Initial boiling point and boiling range-436 F -42 C 147 psia Flash point-1552 F -1040 C Evaporation rate Not applicable. Melting pointfreezing point-3064 F -188 C Initial boiling point and boiling range-436 F -42 C 147 psia Flash point-1552 F -1040 C Evaporation rate Not applicable. Some fuels and their boiling points at atmospheric pressure. In general blends with smaller. In polar compounds the positive.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of liquid propane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.