Boiling point of kcl
Home » datasheet » Boiling point of kclBoiling point of kcl
Boiling Point Of Kcl. Forming men for the priesthood after the model of Jesus Christ teacher priest and shepherd. It is the main constituent of the salts dissolved in seawater from which it was obtained in ancient. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. Therefore it readily releases potassium.
 Potassium Chloride Wikipedia From en.wikipedia.org
Potassium Chloride Wikipedia From en.wikipedia.org
Forming men for the priesthood after the model of Jesus Christ teacher priest and shepherd. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. This six-channel five colors and one FRET channel real-time PCR instrument combines advanced optical technology with precise temperature control to deliver sensitive reliable detection for singlexplex or multiplex reactions. Potassium imparts a lavender colour to a flame. 760 C 1400 F specific gravity. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in.
This six-channel five colors and one FRET channel real-time PCR instrument combines advanced optical technology with precise temperature control to deliver sensitive reliable detection for singlexplex or multiplex reactions.
Potassium maintains intracellular tonicity is required for nerve conduction cardiac skeletal and smooth muscle contraction production of energy the synthesis of nucleic acids maintenance of blood pressure and normal renal function. 760 C 1400 F specific gravity. This agent has potential antihypertensive effects and when taken as a nutritional. Therefore it readily releases potassium. Properties occurrence and uses. Rock salt common salt or sodium chloride has been known for several thousand years.

Potassium chloride or KCl is an ionic solid. It is soluble in glycerol alkalies water but insoluble in ether. It is the main constituent of the salts dissolved in seawater from which it was obtained in ancient. It is extracted from minerals such as carnallite potash and sylvite. Therefore it readily releases potassium.
 Source: youtube.com
Source: youtube.com
It is in the form of white colour. Therefore it readily releases potassium. Potassium imparts a lavender colour to a flame. Its melting point is about 770 C and the boiling point is 1420 C. 1s 2 2s 2 2p 6 3s 2 3p 5.
 Source: quora.com
Source: quora.com
Pointfreezing point Melting pointrange. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK. Its melting point is about 770 C and the boiling point is 1420 C. Forming men for the priesthood after the model of Jesus Christ teacher priest and shepherd. Potassium metal is soft and white with a silvery lustre has a low melting point and is a good conductor of heat and electricity.
 Source: youtube.com
Source: youtube.com
760 C 1400 F specific gravity. Rock salt common salt or sodium chloride has been known for several thousand years. 34 C 29 F density 1 atm 0 C or 32 F 3214 glitre 0429 ouncegallon oxidation states. The CFX96 Touch System is a powerful precise and flexible real-time PCR detection system. 2-8-8-1 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
2-8-8-1 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. It is in the form of white colour. The crystal structure of KCl made up of face-centered cubic FCC unit cells. Pointfreezing point Melting pointrange. KCl being a salt is highly soluble in water.
 Source: youtube.com
Source: youtube.com
G Flash point -170 C 14 F - closed cup h Evaporation rate No data available i Flammability solid gas No data available. It is a source of nitrogen and nitrogen was named after niterPotassium nitrate is one of several nitrogen-containing compounds. Potassium Chloride is a metal halide composed of potassium and chloride. Why can we measure electrical conductivity in our experiment for kcl and substance 2. It has a melting point of 770 C and a boiling point of 1420 C.
 Source: researchgate.net
Source: researchgate.net
Potassium metal is soft and white with a silvery lustre has a low melting point and is a good conductor of heat and electricity. The CFX96 Touch System is a powerful precise and flexible real-time PCR detection system. Properties occurrence and uses. 1s 2 2s 2 2p 6 3s 2 3p 5. It is soluble in glycerol alkalies water but insoluble in ether.

It is in the form of white colour. It is the main constituent of the salts dissolved in seawater from which it was obtained in ancient. 1 1 3 5 7. Potassium chloride or KCl is an ionic solid. Potassium nitrate or Indian Saltpetre is a chemical compound with the chemical formula K N O 3It is an ionic salt of potassium ions K and nitrate ions NO 3 and is therefore an alkali metal nitrateIt occurs in nature as a mineral niter or nitre in the UK.

It has a melting point of 770 C and a boiling point of 1420 C. It is extracted from minerals such as carnallite potash and sylvite. Potassium chloride or KCl is an ionic solid. Potassium maintains intracellular tonicity is required for nerve conduction cardiac skeletal and smooth muscle contraction production of energy the synthesis of nucleic acids maintenance of blood pressure and normal renal function. Potassium chloride is mainly useful in making fertilizers since plants need potassium for their growth and development.
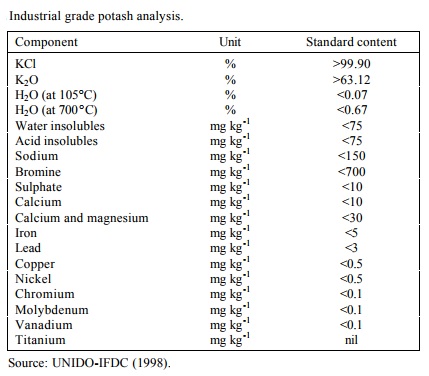 Source: chemicalbook.com
Source: chemicalbook.com
It has a melting point of 770 C and a boiling point of 1420 C. The crystal structure of KCl made up of face-centered cubic FCC unit cells. This six-channel five colors and one FRET channel real-time PCR instrument combines advanced optical technology with precise temperature control to deliver sensitive reliable detection for singlexplex or multiplex reactions. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. Pointfreezing point Melting pointrange.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of kcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.