Boiling point of hydrogen bromide
Home » datasheet » Boiling point of hydrogen bromideBoiling point of hydrogen bromide
Boiling Point Of Hydrogen Bromide. Anthoine Balard in 1826. Special Hazards of Combustion Products. At the boiling point molecules anywhere in the liquid may be vaporized. MW 92 bp 111 C 2318 F.
Trends In Boiling Temperature Of The Hydrogen Halides The Student Room From thestudentroom.co.uk
MW 92 bp 111 C 2318 F. The primary aim of the cards is to promote the safe use of chemicals in the workplace. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. 71 C 160 F. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Energy of first ionisation.
The boiling point at atmospheric pressure.
301 - 033 P 0045 and the BaxBcl-2 ratio decreased in the model-hydrogen group. 517 - 041 P 0021. 706 g100 mL 0 C 757 g100 mL 25 C 1079 g100 mL 100 C Solubility. 0195 nm -1 Isotopes. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Electronic shell Ar 3d 10 4s 2 4p 5.
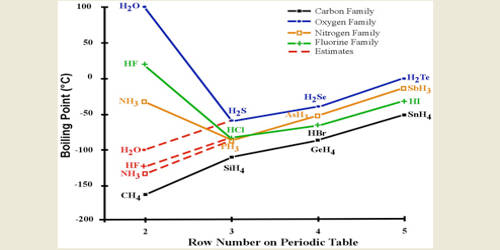 Source: qsstudy.com
Source: qsstudy.com
ALLYL BROMIDE decomposes upon heating and exposure to light forming HBr a strong reducing agent. Boiling Point ºC-05º. 517 - 041 P 0021. 0195 nm -1 Isotopes. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
Source: thestudentroom.co.uk
Methyl ethyl ketone 2. USCG 1999 Health Hazard. Alternatively allyl chloride reacts with hydrogen bromide in the presence. 517 - 041 P 0021. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
Source:
Special Hazards of Combustion Products. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. In the presence of various catalysts such as acids or initiators may undergo exothermic addition polymerization reactions. Methyl chloride Chloromethane 71556. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
USCG 1999 Health Hazard. May form explosive mixtures with air in fire. 1266 K anhydrous decomposes Solubility in water. Physically allyl bromide is a colorless liquid with an intense acrid and persistent smell. Symptoms of exposure include burning sensation coughing wheezing laryngitis shortness of breath headache nausea and vomiting.
Source: quora.com
Hydrogen fluoride Hydrofluoric acid 7783064. Toxic fumes of hydrogen bromide. The ICSC project is a common undertaking between the World Health Organization WHO and. Alternatively allyl chloride reacts with hydrogen bromide in the presence. 71 C 160 F.
 Source: sdk.co.jp
Source: sdk.co.jp
The primary aim of the cards is to promote the safe use of chemicals in the workplace. Irritating to the skin. ALLYL BROMIDE decomposes upon heating and exposure to light forming HBr a strong reducing agent. Reacts violently with oxidizing agents. 517 - 041 P 0021.
Source: quora.com
Irritating to the skin. The boiling point at atmospheric pressure. May form explosive mixtures with air in fire. At the boiling point molecules anywhere in the liquid may be vaporized. Methyl ethyl ketone 2.
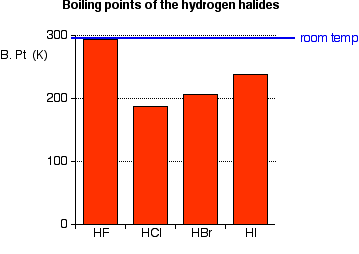 Source: chemguide.co.uk
Source: chemguide.co.uk
Toxic fumes of hydrogen bromide. Energy of first ionisation. 301 - 033 P 0045 and the BaxBcl-2 ratio decreased in the model-hydrogen group. 706 g100 mL 0 C 757 g100 mL 25 C 1079 g100 mL 100 C Solubility. In the presence of various catalysts such as acids or initiators may undergo exothermic addition polymerization reactions.
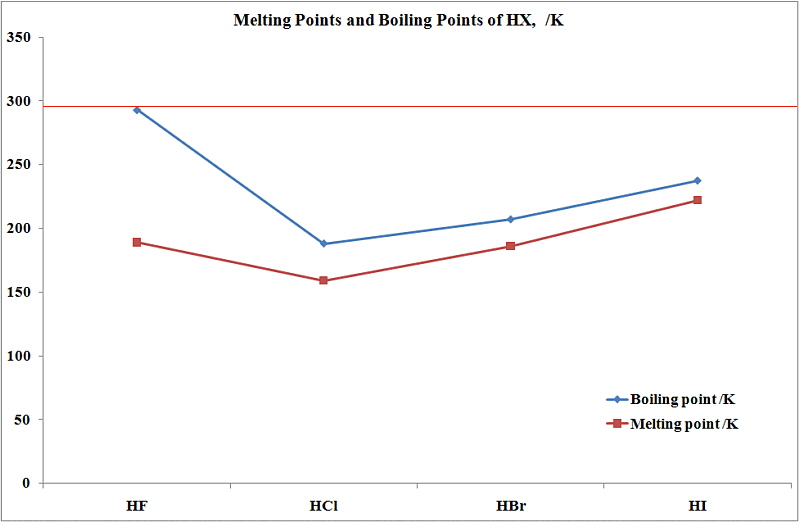 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Hydrogen sulfide See Modification 123319. Water does not normally react with 1º-alkyl halides to give alcohols so the enhanced nucleophilicity of nitrogen relative to oxygen is clearly demonstrated. Electronic shell Ar 3d 10 4s 2 4p 5. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. 0195 nm -1 Isotopes.
Source: en.wikipedia.org
Energy of first ionisation. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Methyl chloride Chloromethane 71556. The main target users are workers and those responsible for occupational safety and health. It is produced commercially from allyl alcohol.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of hydrogen bromide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.