Boiling point of hf
Home » datasheet » Boiling point of hfBoiling point of hf
Boiling Point Of Hf. 2 - Atomic number-253. 12 H 2 O 1 Ionization Potential. Samantha Bidwell Created Date. Boiling points at high pressure.
 Why Does Water Has A Higher Boiling Point Than Hf Hydrogen Bonding In Water And Hydrogen Fluoride Youtube From youtube.com
Why Does Water Has A Higher Boiling Point Than Hf Hydrogen Bonding In Water And Hydrogen Fluoride Youtube From youtube.com
Colorless gas or fuming liquid. 000604 atm 000612 bar 611657 Pa 008871 psi lb f in 2 Triple point temperature. 05 3 ppm caution. Disagreeable pungent odor at less than 1 ppm. People Are Pointing Out Double Standards Theyre Sick Of And This Really Gets My Blood Boiling How its perfectly okay for a potential employer to ask your salary expectations but a. Classified as a transition metal Copper is a solid at room temperature.
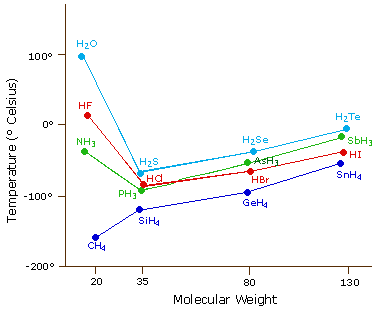
AStrong dipole-dipole bonds between water molecules BStrong hydrogen bonds between water molecules CDispersion forces which are present in all molecules DAsymmetrical shape of the polar bonds.
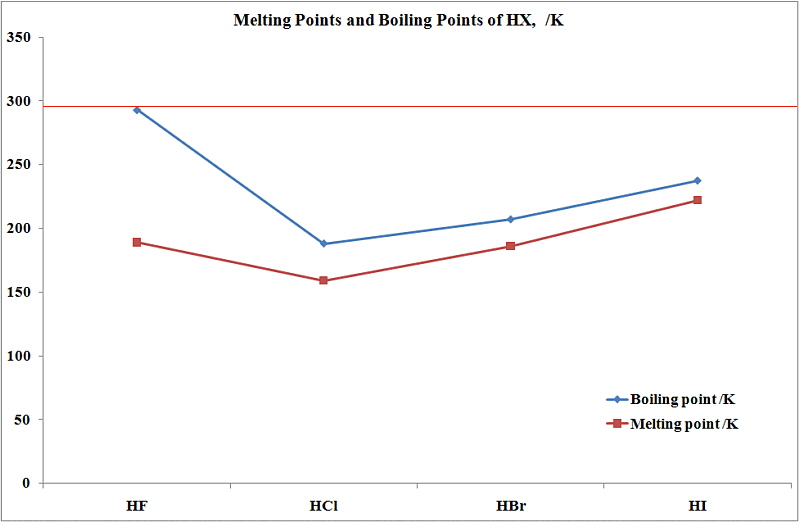
Hf 4876 K 4603 C Ta 5731 K 5458 C W 6203 K 5930 C Re 590015 K 56270 C Os 5285 K 5012 C Ir 4403 K 4130 C Pt 4098 K 3825 C Au 3243 K 2970 C Hg 62988 K 35673 C Tl 1746 K 1473 C Pb 2022 K 1749 C Bi 1837 K. 20132015 Morrill Professor Iowa State University. Copper is a chemical element with symbol Cu and atomic number 29. If your kettle isnt pressurized you wont be able to heat the solution much above the boiling point of water 212 degrees and it might take an additional hour or two to complete the process. ACGIH 2000 8-Hour Time Weighted Average TWA. 68F 20C at 760 mmHg.
 Source: youtube.com
Source: youtube.com
Br 2Br2 59 C and IClICl 97 C Author. 400 mmHg 34F Vapor density. 2015present Senior Instructor II University of Oregon. Samantha Bidwell Created Date. 20132015 Morrill Professor Iowa State University.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1998-2013 Professor of Chemistry Iowa State University. Q4 Give an explanation in terms of intermolecular forces for the following differences in boiling point. 400 mmHg 34F Vapor density. 153 F 67 C Specific Gravity. 20132015 Morrill Professor Iowa State University.
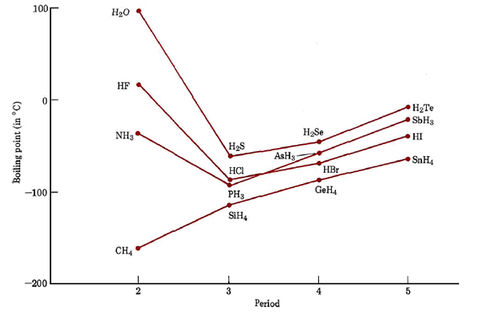 Source: chem.libretexts.org
Source: chem.libretexts.org
12 H 2 O 1 Ionization Potential. 05 3 ppm caution. The elemenents of the periodic table sorted by boiling point. HF is widely used in the petrochemical. 001 C 3202 F.
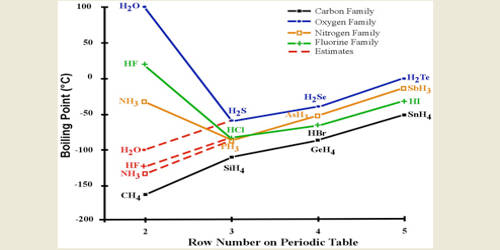 Source: qsstudy.com
Source: qsstudy.com
2006 Visiting Professor University of Arizona. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. We would like to show you a description here but the site wont allow us. What explains the very high melting and boiling point of water. 001 C 3202 F.
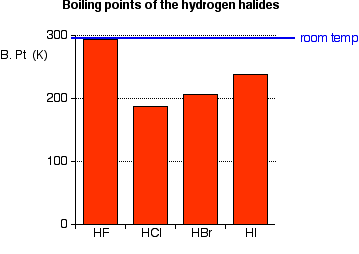 Source: chemguide.co.uk
Source: chemguide.co.uk
2006 Visiting Professor University of Arizona. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. 000604 atm 000612 bar 611657 Pa 008871 psi lb f in 2 Triple point temperature. 68F 20C at 760 mmHg. ACGIH 2000 8-Hour Time Weighted Average TWA.
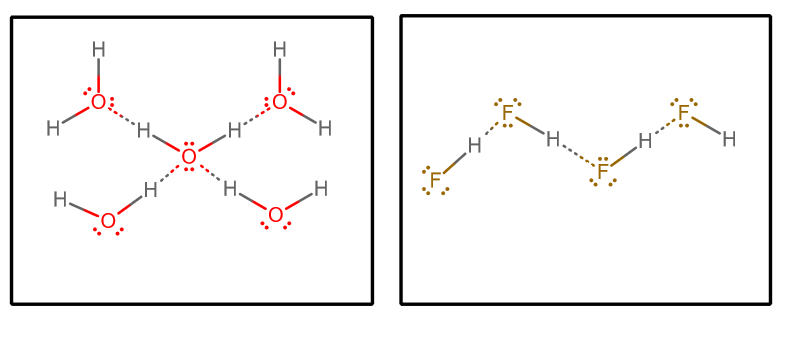 Source: socratic.org
Source: socratic.org
CHCl3CHCl 3 CHBr361 C and CHBr 3 150 C c. HF is widely used in the petrochemical. Reported range is very broad Exposure Limits. If your kettle isnt pressurized you wont be able to heat the solution much above the boiling point of water 212 degrees and it might take an additional hour or two to complete the process. Follow the links below to get values for the listed properties of liquid water at varying pressure and temperature.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
12 H 2 O 1 Ionization Potential. 3 ppm 2. What explains the very high melting and boiling point of water. The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Reported range is very broad Exposure Limits.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
What explains the very high melting and boiling point of water. What explains the very high melting and boiling point of water. Apollo accelerates the growth and success of your entire sales org with the first truly reliable scalable revenue engine and account-based sales platform. Q2 To go. Boiling melting point Viscosity Q1 Rank gas liquid and solid in order of increasing intermolecular forces.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid Colorless gas or fuming liquid below 67F with a strong irritating odor. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and. Apollo accelerates the growth and success of your entire sales org with the first truly reliable scalable revenue engine and account-based sales platform. Reported range is very broad Exposure Limits. Hf 4876 K 4603 C Ta 5731 K 5458 C W 6203 K 5930 C Re 590015 K 56270 C Os 5285 K 5012 C Ir 4403 K 4130 C Pt 4098 K 3825 C Au 3243 K 2970 C Hg 62988 K 35673 C Tl 1746 K 1473 C Pb 2022 K 1749 C Bi 1837 K.
Source: quora.com
Colorless gas or fuming liquid. This list contains the 118 elements of chemistry. CHCl3CHCl 3 CHBr361 C and CHBr 3 150 C c. HFHF 20 C and HClHCl -85 C b. The elemenents of the periodic table sorted by boiling point.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of hf by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.