Boiling point of hbr
Home » datasheet » Boiling point of hbrBoiling point of hbr
Boiling Point Of Hbr. Low melting and boiling points Poor electrical conductors in all. 492 C 918 F. Boiling point - the temperature at which a liquid turns into a gas. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 The Chemistry Of The Halogens 3 From wwwchem.uwimona.edu.jm
The Chemistry Of The Halogens 3 From wwwchem.uwimona.edu.jm
Hydrogen and another non-metal chemically combines through covalent bonding. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Magnetic susceptibility χ 490 10 6 cm 3 mol. Boiling point - the temperature at which a liquid turns into a gas. Chromic acid may also refer to the molecular species H 2 CrO 4 of which the trioxide is the anhydride. 1618 K Solubility in water.
125 mL of 120 M HCl solution is diluted with water to a final volume of 100 LWhat is the molarity of the diluted solution.
1618 K Solubility in water. 146 D Hazards Flash. Boiling point - the temperature at which a liquid turns into a gas. Melting point - the temperature at which a solid turns into a liquid. 1345 C 2453 F. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable.

Refractive index n D 2116 Dipole moment. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable. Melting point - the temperature at which a solid turns into a liquid. Slightly soluble Solubility product K sp 627 10 9. Hydrogen and another non-metal chemically combines through covalent bonding.
 Source: numerade.com
Source: numerade.com
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. 125 mL of 120 M HCl solution is diluted with water to a final volume of 100 LWhat is the molarity of the diluted solution. Hydrogen and another non-metal chemically combines through covalent bonding. By contrast the ionic solid NaCl has a melting point of 800C. 765 K Boiling point.
 Source: qsstudy.com
Source: qsstudy.com
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate which may contain a variety of compounds including solid chromium trioxideThis kind of chromic acid may be used as a cleaning mixture for glass. Magnetic susceptibility χ 490 10 6 cm 3 mol. Usually occurs between non-metals. Soluble in HCl HBr ammonium hydroxide acetonitrile negligible in acetone sulfuric acid. 765 K Boiling point.

By contrast the ionic solid NaCl has a melting point of 800C. Melting point - the temperature at which a solid turns into a liquid. Magnetic susceptibility χ 490 10 6 cm 3 mol. 1345 C 2453 F. 125 mL of 120 M HCl solution is diluted with water to a final volume of 100 LWhat is the molarity of the diluted solution.
 Source: chegg.com
Source: chegg.com
Low melting and boiling points Poor electrical conductors in all. By contrast the ionic solid NaCl has a melting point of 800C. Hydrogen and another non-metal chemically combines through covalent bonding. 3 pts a 375 M b 150 M c 0185 M d 0015 M 23What is the molarity of an NaCl solution prepared by dissolving 93 g of NaCl in 350 mL of. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.

For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. 1618 K Solubility in water. Chromic acid may also refer to the molecular species H 2 CrO 4 of which the trioxide is the anhydride. Its melting point is -23C. 1345 C 2453 F.

Low melting and boiling points Poor electrical conductors in all. Its melting point is -23C. Soluble in HCl HBr ammonium hydroxide acetonitrile negligible in acetone sulfuric acid. Boiling point - the temperature at which a liquid turns into a gas. Usually occurs between non-metals.
 Source: snapsolve.com
Source: snapsolve.com
146 D Hazards Flash. Low melting and boiling points Poor electrical conductors in all. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable. By contrast the ionic solid NaCl has a melting point of 800C. 146 D Hazards Flash.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Chromic acid may also refer to the molecular species H 2 CrO 4 of which the trioxide is the anhydride. Boiling point - the temperature at which a liquid turns into a gas. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Properties of Covalent Compounds Gases liquids or solids made of molecules Atoms share electrons to become stable. 146 D Hazards Flash.
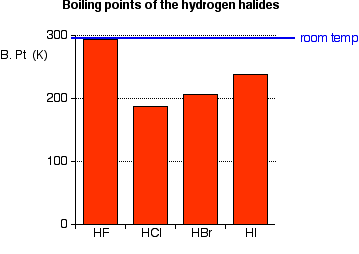 Source: chemguide.co.uk
Source: chemguide.co.uk
By contrast the ionic solid NaCl has a melting point of 800C. Magnetic susceptibility χ 490 10 6 cm 3 mol. 492 C 918 F. Refractive index n D 2116 Dipole moment. 1618 K Solubility in water.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of hbr by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.