Boiling point of fluorine
Home » datasheet » Boiling point of fluorineBoiling point of fluorine
Boiling Point Of Fluorine. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. 1058 K Boiling point. 257 K Thermochemistry Std enthalpy of. All halogens have 7.
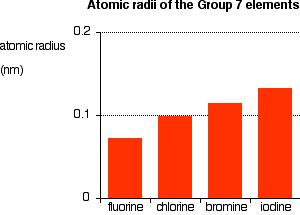 Atomic And Physical Properties Of Periodic Table Group 7 The Halogens From chemguide.co.uk
Atomic And Physical Properties Of Periodic Table Group 7 The Halogens From chemguide.co.uk
Self-extinguishing time fire point is a further. Crystal-like silicon is very brittle. The number designation of each. Not containing fluorine bromine or chlorine atoms. Standard potential - 287 V. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure.
Note that these points are associated with the standard atmospheric pressure.
Unlike the metals the nonmetals do not have universal applications. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. It requires high temperatures to oxidize. If you come across an explanation for the very small increase in melting point from magnesium to aluminium in terms of the strength of the metallic bond you should be very wary of it unless it also explains why despite that the boiling point of aluminium is much higher than that of magnesium. Both the liquid and vapour are poisonous with the liquid form causing deep burns. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 Source: rsc.org
Source: rsc.org
Sir Humphry Davy Louis-Joseph Gay Lussac and Louis-Jacques Thenard all suffered intensely from the effects of inhaling hydrogen fluoride. It exists very rarely as pure silicon in nature. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Sir Humphry Davy Louis-Joseph Gay Lussac and Louis-Jacques Thenard all suffered intensely from the effects of inhaling hydrogen fluoride.
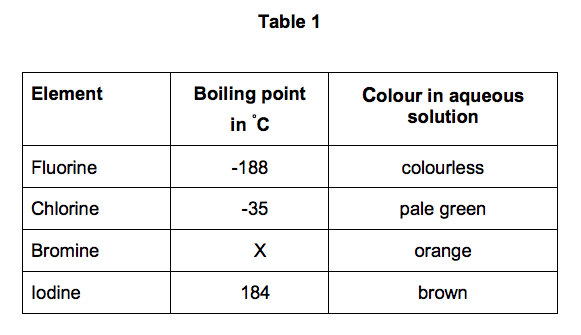 Source: elevise.co.uk
Source: elevise.co.uk
311 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. 16 C 3 F. Davy suffered injury to his eyes and fingernails. The temperature at. P Density g cm 3 0001553.
 Source: chemguide.co.uk
Source: chemguide.co.uk
Essential for life carbon hydrogen nitrogen oxygen sulfur chlorine phosphorus. National Toxicology Program Chemical Repository Database. The agent itself does not burn but it may decompose upon heating to produce corrosive andor toxic fumes. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. And so the mnemonics that students use is FON.
 Source: britannica.com
Source: britannica.com
Type or paste a DOI name into the text box. Actual boiling point is 350C 1. 16 C 3 F. It exists very rarely as pure silicon in nature. Electronic shell He 2s 2 2p 5.
 Source: lizzyfluorine.weebly.com
Source: lizzyfluorine.weebly.com
311 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. It exists very rarely as pure silicon in nature. 311 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Have a look at this table with the elements of the periodic table arranged in order of increasing boiling points. Self-extinguishing time fire point is a further.
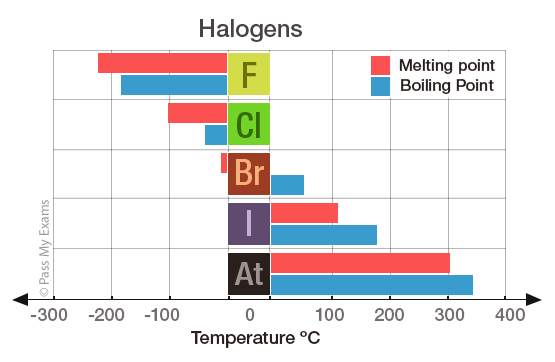 Source: passmyexams.co.uk
Source: passmyexams.co.uk
The boiling point increases moving down the group because the Van der Waals force is greater with increases size and atomic mass. Iron is a metal an element of group VIII of the periodic table. Essential for life carbon hydrogen nitrogen oxygen sulfur chlorine phosphorus. Crystal-like silicon is very brittle. The number designation of each.
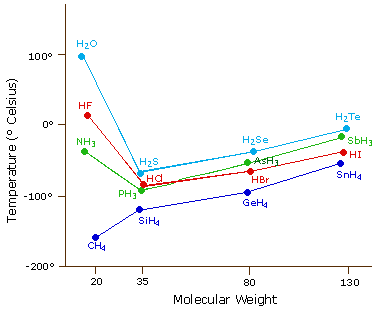 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
Fluorines special status also stems from the fluorine factor the ability of this little atom to fine-tune the chemical properties of an entire molecule. For small fires use dry chemical carbon dioxide or water spray. Alkali Metals Alkaline Earth Metals Transition Metals Other Metals Metalloids Non-Metals Halogens Noble Gases Rare Earth Elements The halogens are five non-metallic elements found in group 17 of the periodic table. Note that these points are associated with the standard atmospheric pressure. The chemical elements of the periodic chart sorted by.
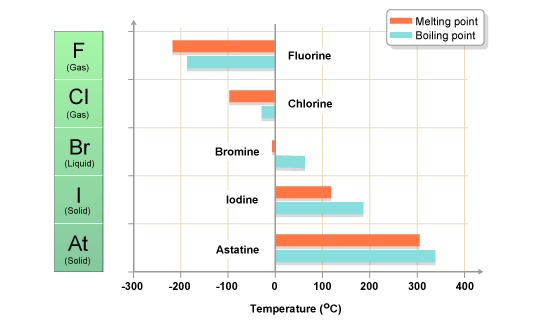 Source: daviddarling.info
Source: daviddarling.info
For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. National Toxicology Program Chemical Repository Database. The boiling point increases moving down the group because the Van der Waals force is greater with increases size and atomic mass. George Knox and his brother.
 Source: slideplayer.com
Source: slideplayer.com
Electron configuration Ar 3d 6 4s 2. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active lifetimes inside the. 16 C 3 F. The boiling point of a substance is the temperature at which this phase change boiling or vaporization occurs. The shorthand convention later introduced to simplify identification of the organic fluorides for a systematic search is used today as the numbering system for refrigerants.
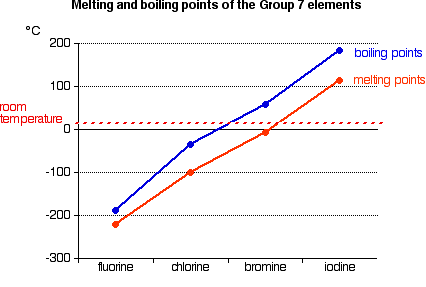 Source: chemguide.co.uk
Source: chemguide.co.uk
Sir Humphry Davy Louis-Joseph Gay Lussac and Louis-Jacques Thenard all suffered intensely from the effects of inhaling hydrogen fluoride. Energy of first ionisation. Data from a variety of non-halogenated ie. For example replacing hydrogen with fluorine can protect drugs from degradation by metabolic enzymes extending their active lifetimes inside the. Type or paste a DOI name into the text box.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of fluorine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.