Boiling point of flourine
Home » datasheet » Boiling point of flourineBoiling point of flourine
Boiling Point Of Flourine. Fluorine is a pale yellow gas that reacts with most substances. And unusually for a noble gas xenon reacts at. Grant and contract funding is sourced from the US National Institutes of Health the Bill Melinda Gates Foundation The Wellcome Trust EDCTP the South African Medical Research Council the National Research Foundation of South. It combines with metals to make fluorides such as sodium fluoride and calcium fluoride both white solids.
 Supplemental Topics From www2.chemistry.msu.edu
Supplemental Topics From www2.chemistry.msu.edu
Essential for life carbon hydrogen nitrogen oxygen sulfur chlorine phosphorus. Sodium fluoride dissolves easily in water but calcium fluoride does not. Fluorine is a chemical element with the symbol F and atomic number 9. Energy of first ionisation. Boiling point - the temperature at which a liquid turns into a gas. 2 - Atomic number-253.
National Toxicology Program Chemical Repository Database.
Above 200 C OF 2 decomposes to oxygen and fluorine via a radical mechanism. Lower melting point and boiling points when compared to metals gain electrons in reactions have a negative oxidation state often colorful in solid state. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas. Energy of first ionisation. 14475 C 22855 F. OF 2 reacts with many metals to yield oxides and fluorides.
 Source: britannica.com
Source: britannica.com
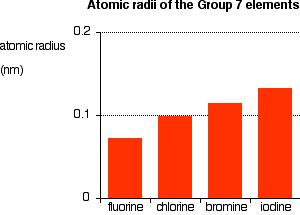
Another physical property that varies across a period is the melting point of the corresponding halide. Fluorine is a pale yellow gas that reacts with most substances. 0136 nm -1. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Unlike the metals the nonmetals do not have universal applications.
 Source: chemguide.co.uk
Source: chemguide.co.uk
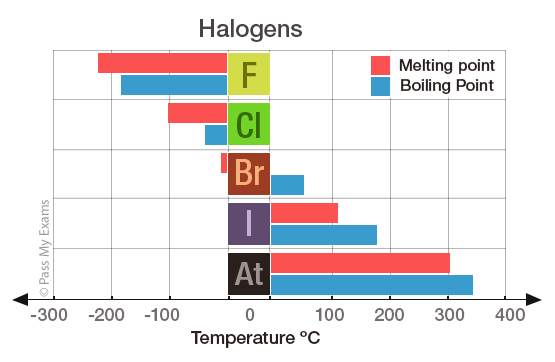
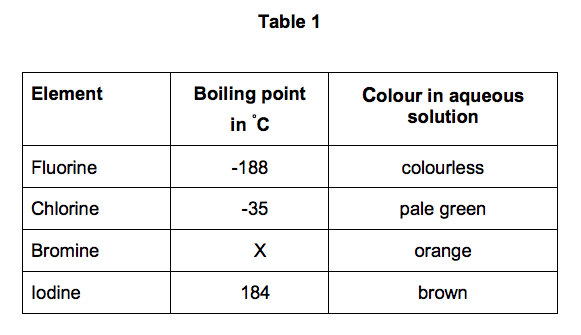
And unusually for a noble gas xenon reacts at. National Toxicology Program Chemical Repository Database. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. The free element melts at 220 C and boils at 188 C. But they do appear together in certain applications.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
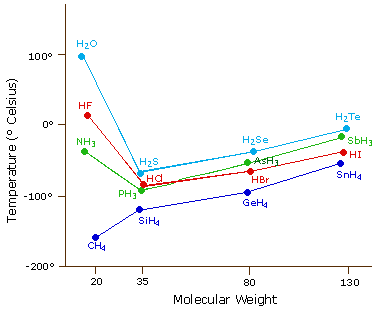
The remaining examples in the table conform to the correlation of boiling point with total electrons and number of nuclei but fluorine containing molecules remain an exception. The temperature at which a liquid boils with the vapor pressure equal to the given external pressure. Through wind-blown soil fluorides are released into the air. Atomic number - Name alphabetically-269. A higher molality will increase the boiling point and decrease the freezing point of the solution.
 Source: slideplayer.com
Source: slideplayer.com
Fire may produce irritating corrosive andor toxic gases. The Earths crust contains 950 parts per million of fluorine. The remaining examples in the table conform to the correlation of boiling point with total electrons and number of nuclei but fluorine containing molecules remain an exception. Research in the IDM is led by over 34 independent principal investigators in the basic clinical and public health sciences and has a strong translational focus. Cold thallium ignites on contact with fluorine.
 Source: passmyexams.co.uk
Source: passmyexams.co.uk
Fluorine is a pale yellow gas that reacts with most substances. ILO International Chemical Safety Cards ICSC-050 C. It is a. 14475 C 22855 F. Classify each of the following changes as physical or chemical.
 Source: rsc.org
Source: rsc.org
The remaining examples in the table conform to the correlation of boiling point with total electrons and number of nuclei but fluorine containing molecules remain an exception. Unlike the metals the nonmetals do not have universal applications. The elemenents of the periodic table sorted by boiling point. Another physical property that varies across a period is the melting point of the corresponding halide. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: lizzyfluorine.weebly.com
Source: lizzyfluorine.weebly.com
12840 K Solubility in water. Fluorine occurs naturally in the crust of the earth where it is present in rocks coal and clay. Sodium fluoride dissolves easily in water but calcium fluoride does not. The agent itself does not burn but it may decompose upon heating to produce corrosive andor toxic fumes. As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium.
 Source: elevise.co.uk
Source: elevise.co.uk
Melting Points of the Halides. Melting Points of the Halides. The temperature at which a liquid boils with the vapor pressure equal to the given external pressure. Fire may produce irritating corrosive andor toxic gases. Fluorine can form ionic bonds with some elements such as carbon and boron and neon does not tend to form any bonds at all.
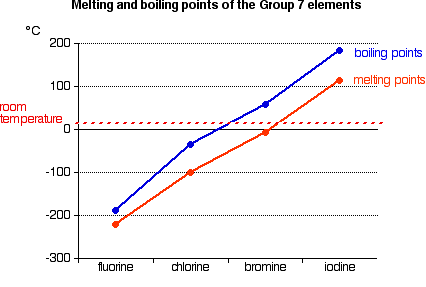 Source: chemguide.co.uk
Source: chemguide.co.uk
Sodium fluoride dissolves easily in water but calcium fluoride does not. ChemSpider is a free chemical structure database. Sulfur gives SO 2 and SF 4. Density g cm 3 78 gcm-3 at 20C. Fluorine is the 13th most abundant element in the crust of the Earth.
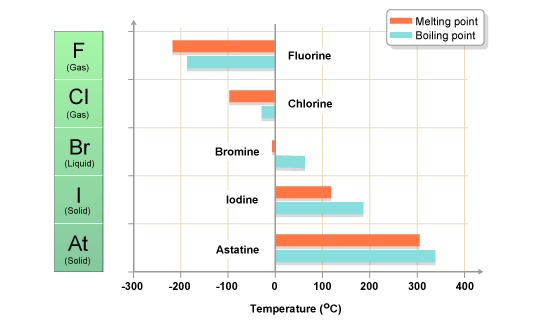 Source: daviddarling.info
Source: daviddarling.info
Standard potential - 287 V. A halide is a. Most of their boiling points are higher than the ten electron compounds neon and methane but fluorine is an exception boiling 25º below methane. Fluorine is a naturally-occurring pale yellow-green gas with a sharp odor. The number designation of each.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of flourine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.