Boiling point of ethylene glycol
Home » datasheet » Boiling point of ethylene glycolBoiling point of ethylene glycol
Boiling Point Of Ethylene Glycol. VAPOR DENSITY air1. Because of its high boiling point and affinity for water ethylene glycol is a useful desiccant. 1115 2020 c WATER SOLUBILITY. Consult standard literature for details of treatment.
 Phase Equilibrium Diagram Of Ethylene Glycol Water Mixtures At Download Scientific Diagram From researchgate.net
Phase Equilibrium Diagram Of Ethylene Glycol Water Mixtures At Download Scientific Diagram From researchgate.net
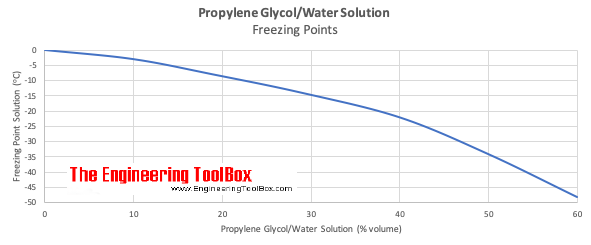
Freezing Points of Propylene Glycol based Water Solutions. Soluble in many alcohols ketones ethers esters. Antifreeze humectant plasticiser hydraulic fluid solvent IN OTHER SOLVENTS. Not listed by ACGIH IARC NTP or CA Prop 65. 371 to 373 K 5 mmHg Hazards GHS pictograms. There are a number of benefits using ethylene glycol over propylene glycol especially in closed loop systems were risk of contact with food is minimal.
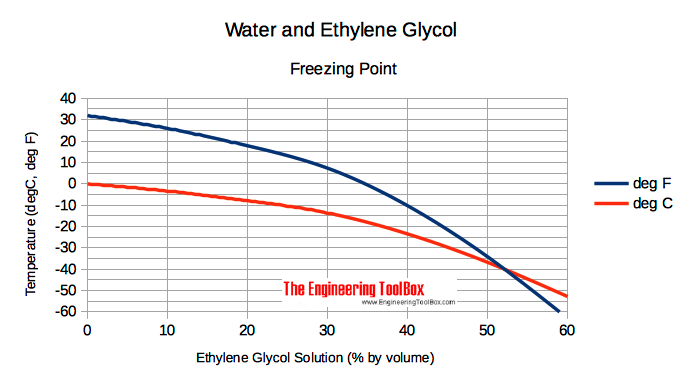
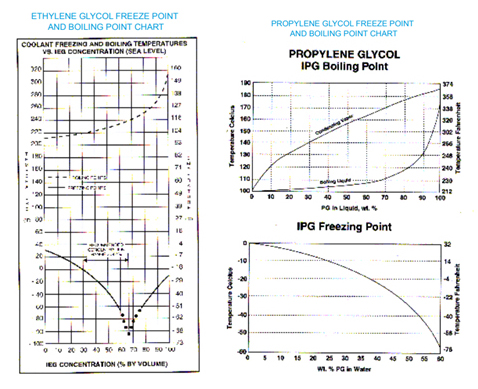
Freezing point 100 ethylene glycol at atmospheric pressure is -128 o C 9 o F 1 Btulb m o F 41868 Jkg K 1 kcalkg o C Note.
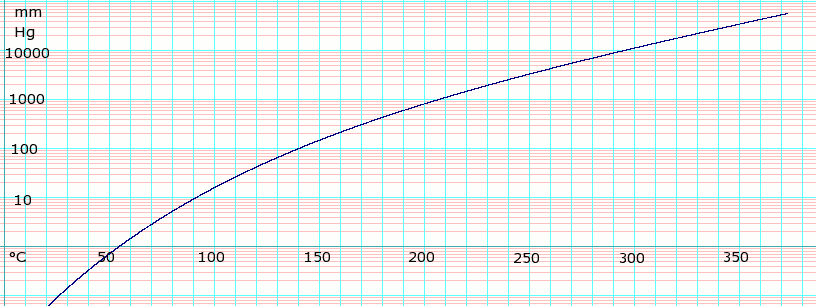
VAPOR DENSITY air1. -13 c SPECIFIC GRAVITY. A liquid boils when its vapour pressure is equal to the atmospheric pressure. The most common antifreeze fluid - ethylene glycol - must not be used where there is a chance of leakage to potable water or food processing systems. 98 to 100 C 208 to 212 F. 3732 K Boiling point of ethanol.

It is produced by heating either natural gas especially its ethane and propane components or petroleum to 800900 C 14701650 F giving a mixture of gases from which the ethylene is separated. Ethylene glycol is used in the natural gas industry to remove water vapor from natural gas before further processing in much the same manner as triethylene glycol TEG. Alcohols boil considerably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen bonding isomers being substantially higher boiling. Immediate steps should be taken to limit its spread to the environment. For example freeze point depression is much more effective using ethylene glycol so more propylene glycol would be required to maintain the same freeze point as ethylene.
 Source: researchgate.net
Source: researchgate.net
The molar mass of ethylene glycol is 6207 gmol. 233 K Boiling point. Ethylene glycol is a colorless liquid with the chemical formula C2H6O2. Dipropylene glycol is a colorless nearly odorless liquid with a high boiling point and low toxicity. Soluble in many alcohols ketones ethers esters.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Boiling point of water. The melting point of ethylene is 1694 C 2729 F and its boiling point is 1039 C 1550 F. 100 C 212 F Boiling point of water in Kelvin. The boiling and freezing points of glycol mixtures are a function of the relative amounts of glycol and water in the mixture. Consult standard literature for details of treatment.
 Source: researchgate.net
Source: researchgate.net
It is odorless but has a sweet. 076 mm Hg at 68 F. -1958 C -3204 F Boiling point of liquid helium. Ethylene glycol is a synthetic liquid substance that absorbs water. Alcohols boil considerably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen bonding isomers being substantially higher boiling.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The ethers of ethylene glycol are used as solvents and plasticizers. 1115 2020 c WATER SOLUBILITY. -107 F -77 C. HOCH 2 CH 2 OH CAS Registry Number. 340 F 171 C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The melting point of ethylene is 1694 C 2729 F and its boiling point is 1039 C 1550 F. VAPOR DENSITY air1. The potential for ignition of solvents in cold cleaning under shipment or storage conditions is assessed by measuring flash point in an open cup tester. The primary hazard is the threat to the environment. The single oral lethal dose for humans has been estimated at 14 mlkg 156 gkg or about 100 ml 111 g for an adult.
 Source: hellafunctional.com
Source: hellafunctional.com
-107 F -77 C. 40 C 40 F. The current practice is to list the alkyl groups in alphabetical order t-butyl methyl ether but older names often list the alkyl groups in increasing order of size methyl t-butyl ether. Boiling point helps identify and characterise a compound. On that basis treatment similar to ethylene glycol intoxication may be of benefit.
 Source: sciencedirect.com
Source: sciencedirect.com
The primary hazard is the threat to the environment. For instance a solution of 10 ethylene glycol freezes at -34 C 259 F 30 ethylene glycol freezes at -137 C 73 F and. Since it is a liquid it can easily penetrate the soil and contaminate groundwater and nearby streams. The melting point of ethylene is 1694 C 2729 F and its boiling point is 1039 C 1550 F. It is produced by heating either natural gas especially its ethane and propane components or petroleum to 800900 C 14701650 F giving a mixture of gases from which the ethylene is separated.
 Source: penray.com
Source: penray.com
233 K Boiling point. Kinetic energy depends on the temperature mass and. The potential for ignition of solvents in cold cleaning under shipment or storage conditions is assessed by measuring flash point in an open cup tester. 40 C 40 F. 371 to 373 K 5 mmHg Hazards GHS pictograms.
 Source: processecology.com
Source: processecology.com
100 C 212 F Boiling point of water in Kelvin. CH 3 CO 2 H. The boiling point of organic compounds can give important information about their physical properties and structural characteristics. Boiling point helps identify and characterise a compound. Kinetic energy depends on the temperature mass and.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of ethylene glycol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.