Boiling point of ether
Home » datasheet » Boiling point of etherBoiling point of ether
Boiling Point Of Ether. Class IA Flash point below 73 F boiling point below 100 F. Similar behavior can be seen when comparing the boiling point of three isomeric amines. Its boiling point is 35 o C. Solid acetyl peroxide in contact with ether or any volatile solvent may explode violently.
 Quotes About Boiling Point 29 Quotes From quotemaster.org
Quotes About Boiling Point 29 Quotes From quotemaster.org
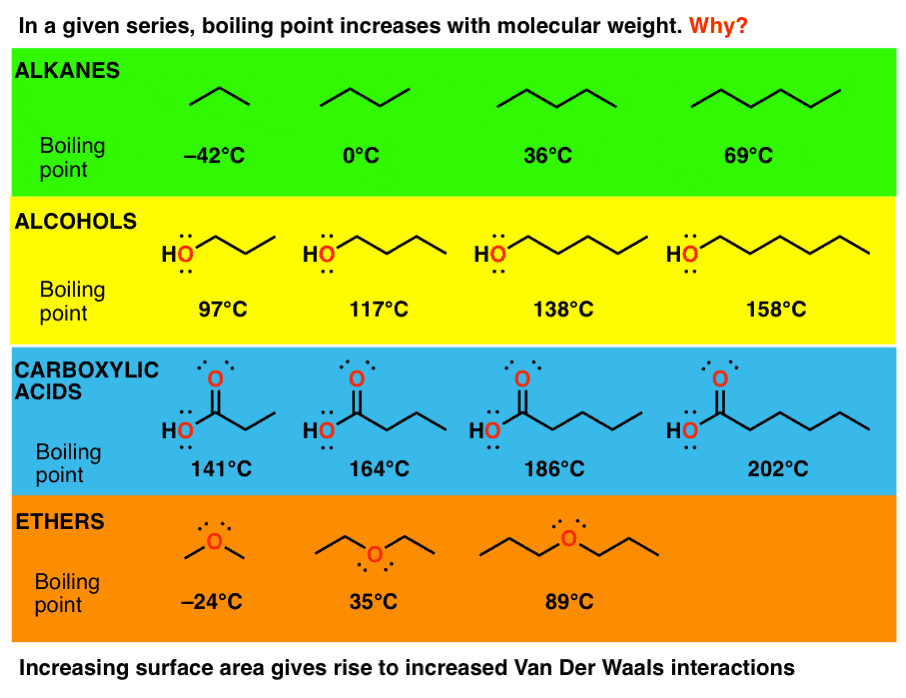
Ether is synthesized by the dehydration of ethanol using sulphuric acid. Further proof as if more was needed that. The C-O bond dipoles reinforce each other so the molecule has a dipole moment. Give reason for the higher boiling point of ethanol in comparison to methoxymethane. Although dipole-dipole forces. If this all seems rather ambiguous contradictory and imprecise well you have a point.
The very lightest most volatile liquid hydrocarbon solvents.
Comments on the method. The boiling point of n-butanol is 117 o C. Must ALWAYS be stored in approved flammable storage cabinet re. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol. 1927C 3789F Specific Gravity. Boiling Point Elevation and Freezing Point Depression The figure below shows the consequences of the fact that solutes lower the vapor pressure of a solvent.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
I The alkoxy. The boiling point of n-butanol is 117 o C. Petroleum ether is the petroleum fraction consisting of aliphatic hydrocarbons and boiling in the range 3560 C and commonly used as a laboratory solvent. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Dipole-dipole forces are not as strong as hydrogen bonds so dimethyl ether has a lower boiling point than methanol does.
 Source: quora.com
Source: quora.com
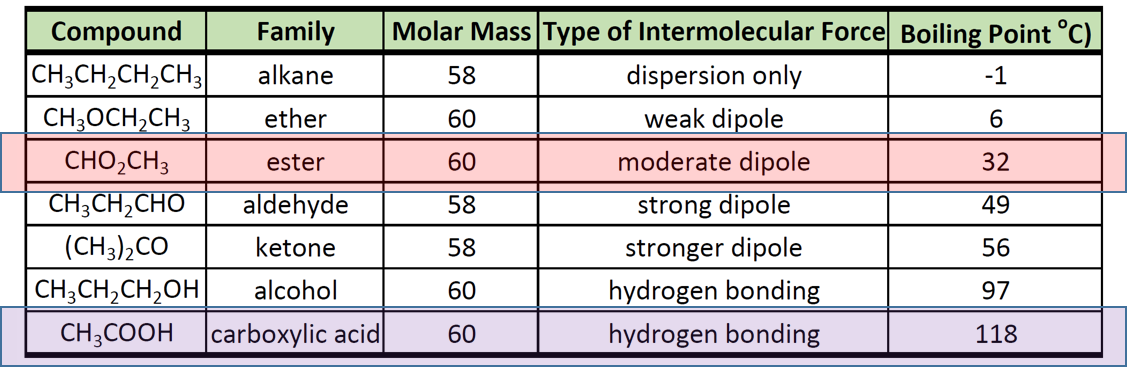
CH 3 CH 2 OH. 2040 595 179 40 K f. Similar behavior can be seen when comparing the boiling point of three isomeric amines. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. 2989 44 39 Acetic acid.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Although dipole-dipole forces. 083 ppm WaterOil Dist. Give reason for the higher boiling point of ethanol in comparison to methoxymethane. Solid acetyl peroxide in contact with ether or any volatile solvent may explode violently. 562 167 948 K b.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Ii directs the incoming substituents towards ortho and para positions in the ring. Its not a straightforward topic. Gardless of amount. Highly branched vs. Although dipole-dipole forces.
 Source: britannica.com
Source: britannica.com
The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. Ethyl alcohol C 2 H 6 O The normal boiling point of ethyl alcohol is 785 o C ie a liquid at room temperature. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol. 2CH 3 CH 2 OH 2H 2 SO 4 CH 3 CH 2 2 O H 2 SO 4 H 2 O. PEEKs chemical name is Poly oxy-1 4-phenyleneoxy-1 4-phenylenecarbonyl-1 4-phenylene.
 Source: studychemistrytopics.tumblr.com
Source: studychemistrytopics.tumblr.com
This is quite a complex preparation and showing the video prior to the experiment definitely helps. CH 3 CH 2 2 NH 2. CH 3 CH 2 NHCH 3. Its boiling point is 35 o C. Boiling Point Melting Point.
 Source: wou.edu
Source: wou.edu
083 ppm WaterOil Dist. Its not a straightforward topic. A mixture of ether and ozone forms aldehyde and acetic acid and a heavy liquid. Compound Formula C 2 H 5 2 O. Trimethylamine CH 3 3 N.
 Source: quotemaster.org
Source: quotemaster.org
Its boiling point is 35 o C. Despite the name petroleum ether is not classified as an ether. If this all seems rather ambiguous contradictory and imprecise well you have a point. Often incorporated into latex emulsion coatings. You can now roughly evaluate its boiling point.

Often incorporated into latex emulsion coatings. Now you can calculate its boiling point under any pressure. Solid acetyl peroxide in contact with ether or any volatile solvent may explode violently. If this all seems rather ambiguous contradictory and imprecise well you have a point. The relatively weak dipole-dipole forces and London dispersion forces between molecules results in a much lower normal boiling point compared to ethyl alcohol.
 Source: youtube.com
Source: youtube.com
The ether is removed during the final distillation and the first distillate coming over below 73oC should be discarded. Ii directs the incoming substituents towards ortho and para positions in the ring. Compare its boiling point with that of n-butanol. The ester functional group has a similar character to the ketone and aldehyde functional group. Despite the name petroleum ether is not classified as an ether.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of ether by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.