Boiling point of ethene
Home » datasheet » Boiling point of etheneBoiling point of ethene
Boiling Point Of Ethene. Ethylene is widely used. In contrast natural gas methane has a boiling point of -1615C -2587F at atmospheric pressure. It is the simplest alkene a hydrocarbon with carbon-carbon double bonds. Most molecular substances are insoluble or only very sparingly soluble in.
 Ch105 Chapter 8 Alkenes Alkynes And Aromatic Compounds Chemistry From wou.edu
Ch105 Chapter 8 Alkenes Alkynes And Aromatic Compounds Chemistry From wou.edu
However ethene is an exception because it is a colourless gas but has a faintly sweet odour. Most molecular substances are insoluble or only very sparingly soluble in. Crisco by partial hydrogenation of their unsaturated components. 1037 C 1547 F. Addition of an entraining agent such as benzene cyclohexane or heptane allows a new ternary azeotrope comprising the ethanol water and the entraining agent to be formed. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation.
But at around 260 degrees diesel condenses out of the gas.
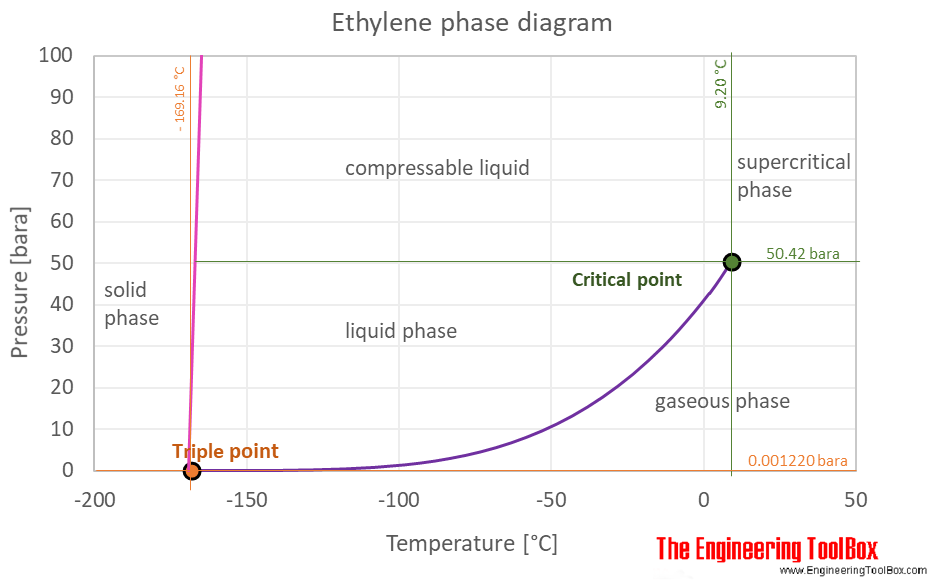
These double-bonded compounds are colourless and odourless in nature. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg. Addition of an entraining agent such as benzene cyclohexane or heptane allows a new ternary azeotrope comprising the ethanol water and the entraining agent to be formed. The size of the melting or boiling point will depend on the strength of the intermolecular forces. The curve between the critical point and the triple point shows the ethylene boiling point with changes in pressure. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
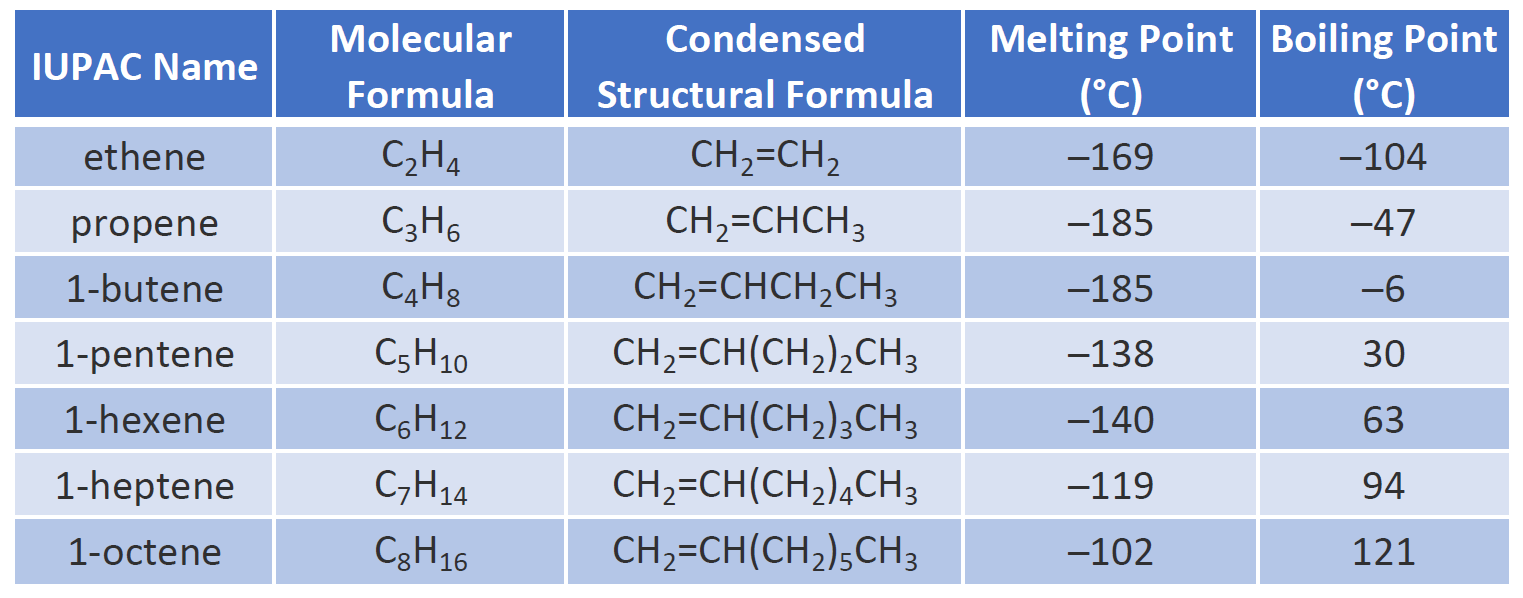
It is a colorless flammable gas with a faint sweet and musky odor when pure. These double-bonded compounds are colourless and odourless in nature. Ethylene is widely used. Natural mixed triglycerides have somewhat lower melting points the melting point of lard being near 30 º C whereas olive oil melts near -6 º C. Ethene and Propene are the first two hydrocarbons.
 Source: thermopedia.com
Source: thermopedia.com
Crisco by partial hydrogenation of their unsaturated components. Ethylene is widely used. However ethene is an exception because it is a colourless gas but has a faintly sweet odour. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. The larger the molecule the more van der Waals attractions are possible - and those will also need more energy to break.
 Source: researchgate.net
Source: researchgate.net
Ethene and Propene are the first two hydrocarbons. At around 180 degrees kerosene. Citation needed 29 mgL. The precise details are different at every refinery and depend on the type of crude oil being distilled. Some of the remaining.
 Source: chemistryscl.com
Source: chemistryscl.com
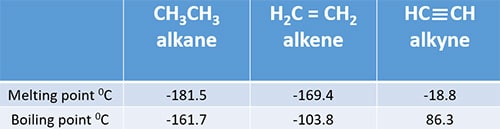
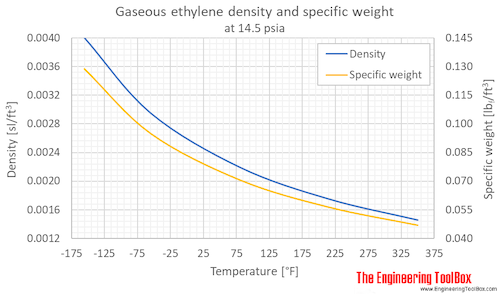
The larger the molecule the more van der Waals attractions are possible - and those will also need more energy to break. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg. The presence of hydrogen bonding will lift the melting and boiling points. Physical Properties of Alkenes.
 Source: wou.edu
Source: wou.edu
1695 K Solubility in water. Physical Properties of Alkenes. But at around 260 degrees diesel condenses out of the gas. Ethene is a hydrocarbon which has the formula C 2 H 4 or H 2 CCH 2. The precise details are different at every refinery and depend on the type of crude oil being distilled.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
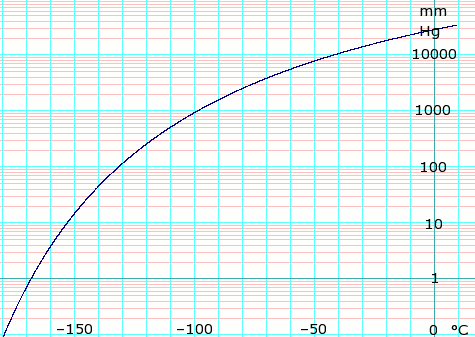
However ethene is an exception because it is a colourless gas but has a faintly sweet odour. The curve between the critical point and the triple point shows the ethylene boiling point with changes in pressure. Different hydrocarbons condense out of the gas cloud when the temperature drops below their specific boiling point. However ethene is an exception because it is a colourless gas but has a faintly sweet odour. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. The precise details are different at every refinery and depend on the type of crude oil being distilled. The curve between the critical point and the triple point shows the ethylene boiling point with changes in pressure. At the critical point there is no change of state when pressure is increased or if heat is added. 1695 K Solubility in water.

It is a colorless flammable gas with a faint sweet and musky odor when pure. 1037 C 1547 F. The first three members of the alkene group are gaseous in nature the next fourteen members are liquids and the remaining alkenes. The higher the gas rises in the tower the lower the temperature becomes. Addition of an entraining agent such as benzene cyclohexane or heptane allows a new ternary azeotrope comprising the ethanol water and the entraining agent to be formed.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Propane stays liquid above the propane boiling point because it is under pressure in a gas cylinder. These double-bonded compounds are colourless and odourless in nature. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. Ethylene is widely used. 1695 K Solubility in water.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The higher the gas rises in the tower the lower the temperature becomes. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. At around 180 degrees kerosene. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg. Propane boiling point is -42C or -44F at atmospheric pressure the point at which liquid propane vaporises into gaseous propane.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of ethene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.