Boiling point of ethane thiol
Home » datasheet » Boiling point of ethane thiolBoiling point of ethane thiol
Boiling Point Of Ethane Thiol. You may also read. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. What volume of 2 M KOH would be required to equivalence point after boiling. 125 mL of a solution of tribasic acid molecular weight 210 was.
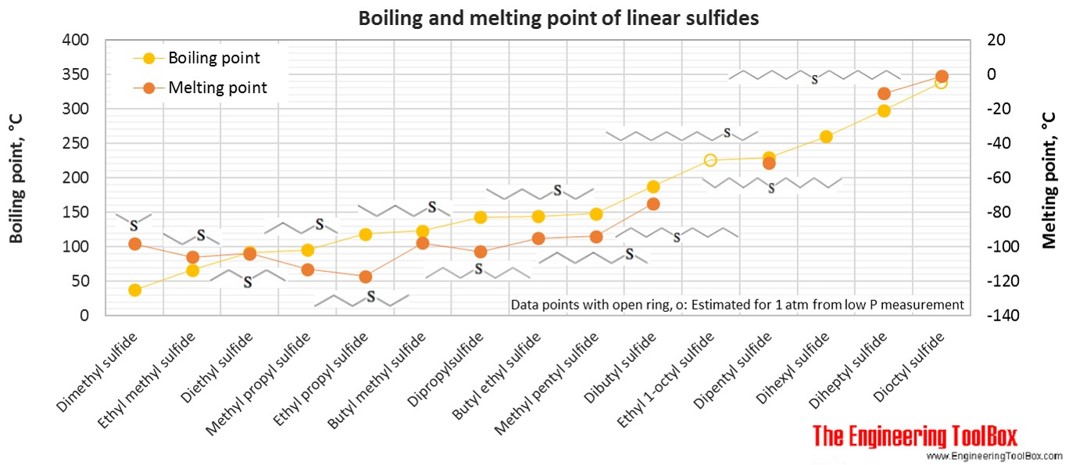 Organic Sulfur Compounds Physical Data From engineeringtoolbox.com
Organic Sulfur Compounds Physical Data From engineeringtoolbox.com
1 methane 2 ethane 3 propane 4 butane 5 pentane 6 hexane 7 heptane 8 octane 9 nonane 10 decane. 76000 mm Hg Vapor Pressure. Which of the following statement is correct. A Fibrous proteins are generally soluble in water b Albumin is an example of fibrous proteins c In fibrous. Ethyl 2-hydroxyethyl sulfide FL. Straight chain alkane names.
Furfuryl 2-methyl-3-furyl disulfide FL.
It has a role as a rodenticide. Sf4 2 lewis structure email protected. Alcohols can also engage in hydrogen bonding with water molecules Figure 23 Hydrogen Bonding between Methanol Molecules and Water Molecules. Development of Atomic Theory. Boiling Point C Feature. Development Of Modern Periodic Table.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The boiling point of the alcohol ethanol is 7829 C compared to 69 C for the hydrocarbon hexane and 346 C for diethyl ether. You may also read. The main target users are workers and those responsible for occupational safety and health. Diethyl ether is also used as a common solvent at a low temperature boiling point 346 C and was used as an anesthetic agent. An organic compound composed of carbon and.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Determination Of Ph Of Some Solutions Experiment. Deviation From Ideal Gas Behaviour. Determination Of Melting Point Of An Organic Compound. Alcohols can also engage in hydrogen bonding with water molecules Figure 23 Hydrogen Bonding between Methanol Molecules and Water Molecules. Drawing the lewis structure for hcn.
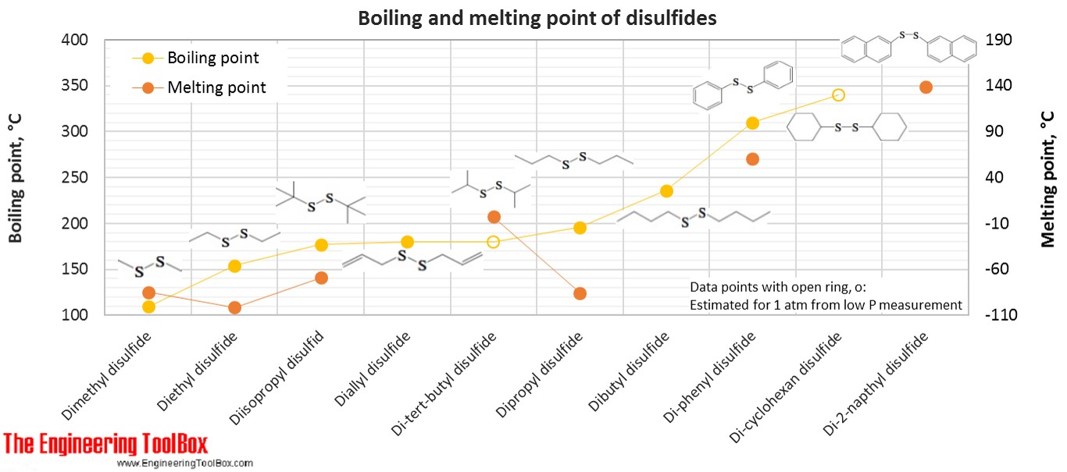 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Tetrahidofuran is cyclic Ether one of the most polar ether used as a solvent. Furfuryl 2-methyl-3-furyl disulfide FL. Determination Of Ph Of Some Solutions Experiment. Thus whereas the hydrocarbons are insoluble in water. The carbon atoms in saturated hydrocarbons _____.
 Source: chegg.com
Source: chegg.com
Polyethylene glycol is a linear polyether is used in cosmetics and pharmaceuticals. What volume of 2 M KOH would be required to equivalence point after boiling. The carbon atoms in saturated hydrocarbons _____. It is added to odorless gaseous products such as liquefied petroleum gas LPG to provide a garlic scent which helps warn of gas leaks. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
Limits M-factors Notes ATP insertedATP Updated Hazard Class and Category Codes. An organic compound composed of carbon and. Occurrence in nature. In the first step the nucleophile which is the pair of electrons in the Cu-CH 3 bond NOT the negative charge on copper forms a bond with the beta position of the ketone. Development Of Modern Periodic Table.
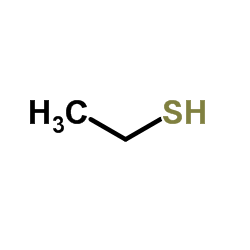 Source: chemsrc.com
Source: chemsrc.com
An organic compound composed of carbon and. For example compare isobutane 2-methylpropane and n-butane butane which boil at 12 and 0 C and 22-dimethylbutane and 23-dimethylbutane which boil at 50 and 58 C respectively. Enter the email address you signed up with and well email you a reset link. An organic compound composed of carbon and. Dialysis Of Lyophilic And Lyophobic Sol.
Source: chemspider.com
Ethane dithiol 1 in ethanol 945 ethyl acetate 4 FL. 1 methane 2 ethane 3 propane 4 butane 5 pentane 6 hexane 7 heptane 8 octane 9 nonane 10 decane. A straight-chain alkane will have a boiling point higher than a branched-chain alkane due to the greater surface area in contact thus the greater van der Waals forces between adjacent molecules. The boiling point of the alcohol ethanol is 7829 C compared to 69 C for the hydrocarbon hexane and 346 C for diethyl ether. Tetrahidofuran is cyclic Ether one of the most polar ether used as a solvent.
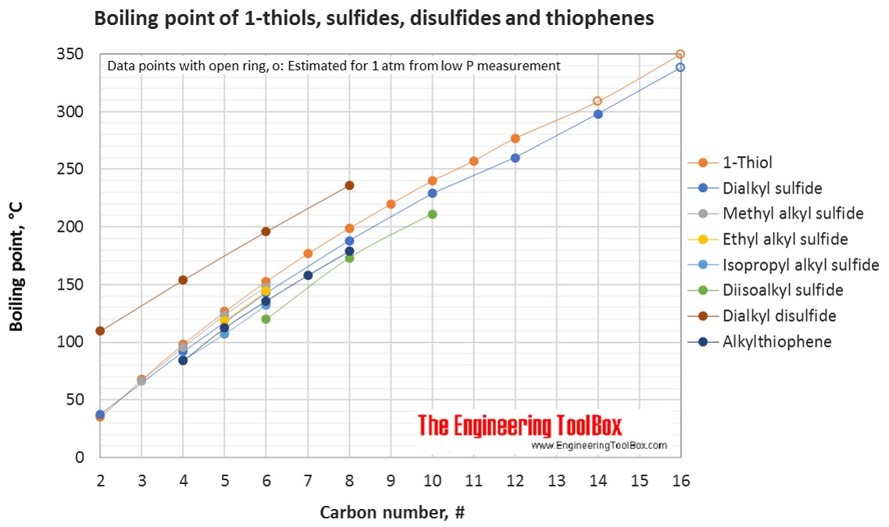 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The C-C π bond breaks forming a negative charge on the alpha carbon. You may also read. Ethane dithiol 1 in ethanol 945 ethyl acetate 4 FL. CH 3 CH 2 CH 2 OH. It is added to odorless gaseous products such as liquefied petroleum gas LPG to provide a garlic scent which helps warn of gas leaks.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Dialysis Of Lyophilic And Lyophobic Sol. Development of Atomic Theory. In the first step the nucleophile which is the pair of electrons in the Cu-CH 3 bond NOT the negative charge on copper forms a bond with the beta position of the ketone. Has a low boiling point c. Meat flavors FL.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The carbon atoms in saturated hydrocarbons _____. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. Determination Of Melting Point Of An Organic Compound. To calculate the Isoelectric point of an amino acid or peptide or protein if you are daring you take the pKa of the carboxylic acid between 2 and 4 usually and the amine 9-11 add them and divide by 2 for the case of an amino acid with no side chain that has a pKa value. Point Menthol MeOH Mercaptan Meso Meso compound.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of ethane thiol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.