Boiling point of ethane
Home » datasheet » Boiling point of ethaneBoiling point of ethane
Boiling Point Of Ethane. Fuel liquid methane natural gas phase phase diagram pressure propane propane storage propane tanks vapor pressure. When methyl bromide or methyl iodide and sodium are heated in the presence of dry ether ethane is formed. As with boiling points the melting point of a solid is dependent on the strength of those attractive forces. C 6 H 14-95.
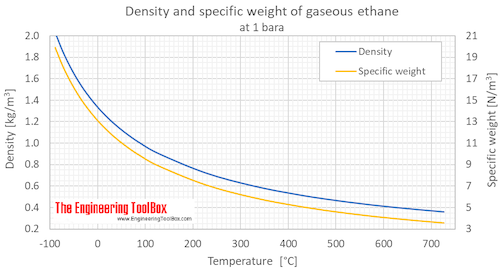 Ethane Density And Specific Weight From engineeringtoolbox.com
Ethane Density And Specific Weight From engineeringtoolbox.com
NGLs include ethane propane butane iso-butane and natural gasoline. Ethane is synthesized by reduction of ethyl iodide using zinc copper couple in alcohol. C 11 H 24-25. C 5 H 12-130. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid. Get the latest odds spreads and betting lines from this weeks games as well as full coverage of the National Football League from USA TODAY.
Ethylene glycol IUPAC name.
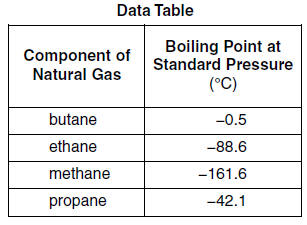
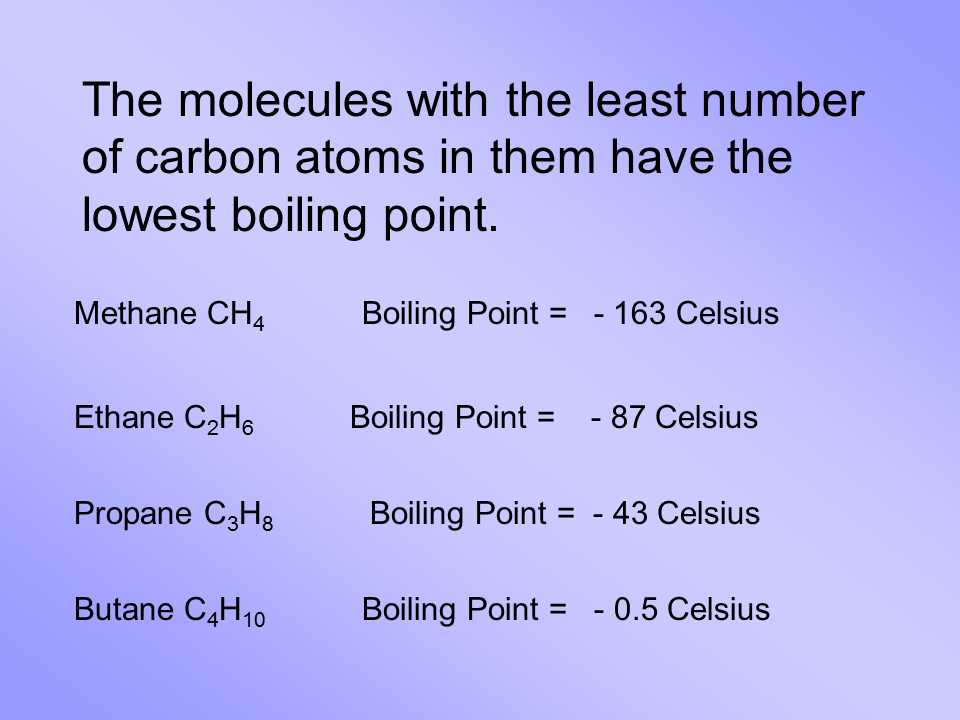
3 Chemical and Physical Properties Expand this section. Boiling Point C Vapor Pressure at 21C kPa Methane CH 4-162. While the ethane propane butane and pentanes must be removed from natural gas this does not mean that they are all waste products. C 10 H 22-30. Hexane C 6 H 14. 54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram.
 Source: youtube.com
Source: youtube.com
The melting point is the temperature at which a solid changes into a liquid. It has a role as a GABA agonist. Ethane ˈ ɛ θ eɪ n or ˈ iː θ eɪ n is an organic chemical compound with chemical formula C 2 H 6At standard temperature and pressure ethane is a colorless odourless gasLike many hydrocarbons ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refiningIts chief use is as feedstock for ethylene production. Density 75 lb gal. It derives from a hydride of an ethane.
 Source: thermopedia.com
Source: thermopedia.com
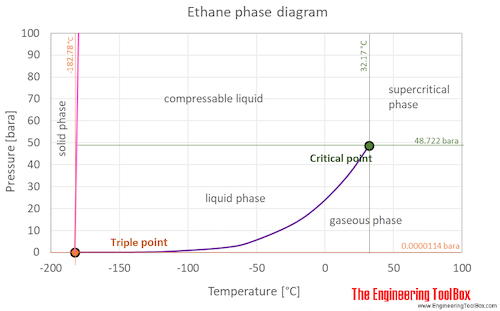
At the critical point there is no change of state when pressure is increased or if heat is added. Boiling Point C Feature. C 30 H 62. It is an odorless colorless sweet-tasting toxic viscous liquid. Ethane is synthesized by reduction of ethyl iodide using zinc copper couple in alcohol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is mainly used for two purposes as a raw material in the manufacture of polyester fibers and for antifreeze formulations. Iodoethane is an iodoalkane that is ethane substituted by an iodo group. In fact associated hydrocarbons known as natural gas liquids NGLs can be very valuable by-products of natural gas processing. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. It also shows the saturation pressure with changes in temperature.
 Source: kentchemistry.com
Source: kentchemistry.com
NGLs include ethane propane butane iso-butane and natural gasoline. It also shows the saturation pressure with changes in temperature. C 3 H 8-190-42. Fuel liquid methane natural gas phase phase diagram pressure propane propane storage propane tanks vapor pressure. It has a role as a GABA agonist.
 Source: youtube.com
Source: youtube.com
These NGLs are sold separately and have a. Boiling Point C Feature. Fuel liquid methane natural gas phase phase diagram pressure propane propane storage propane tanks vapor pressure. C 9 H 20-51. Butane C 4 H 10.
 Source: alevelchem.com
Source: alevelchem.com
To submit a. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. Density 75 lb gal. Ethane is also prepared by Wurtz reaction. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid.

At the boiling point molecules anywhere in the liquid may be vaporized. Flash point of 91F and a melting point of 47F. Density 75 lb gal. When methyl bromide or methyl iodide and sodium are heated in the presence of dry ether ethane is formed. C 8 H 18-57.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
2 Names and Identifiers Expand this section. Vapors are heavier than air. Flash point of 91F and a melting point of 47F. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. At the boiling point molecules anywhere in the liquid may be vaporized.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It derives from a hydride of an ethane. C 7 H 16-91. Ethane is also prepared by Wurtz reaction. The curve between the critical point and the triple point shows the acetone boiling point with changes in pressure. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F.
The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. Ethylenediamine is an alkane-alphaomega-diamine in which the alkane is ethane. Heptane C 7 H 16. 54 Likes 13 Comments - UCLA VA Physiatry Residency uclava_pmrresidency on Instagram. It derives from a hydride of an ethane.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of ethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.