Boiling point of dmf
Home » datasheet » Boiling point of dmfBoiling point of dmf
Boiling Point Of Dmf. Above enter the boiling point under the other pressure. Set up for vacuum distillation. A combination of the two. So that the greenness of the solvents can be ranked on a fair like-for-like basis a cluster analysis grouped similar solvents together.
 Boiling Points Of Components And Azeotropic Composi Tion Download Scientific Diagram From researchgate.net
Boiling Points Of Components And Azeotropic Composi Tion Download Scientific Diagram From researchgate.net
This is a reagent required for this method of LSD production. Carbon tet is great if you can find it. Evaporation DMF aniline toluene CALIBRATION. DMF DMSO DNA DNA alkylator. British Pharmacopoeia BPH Bachelor of Public Health benign prostatic hyperplasia Bq becquerel SI unit of radionuclide activity Br bromine BS BSc Bachelor of Science Baccalaureus Scientiae BSA body surface area BSN Bachelor of Science in Nursing BT bleeding time BTPS body temperature ambient pressure saturated with water vapor BTU British thermal unit. In addition to the above you can use special boiling point SBP solvent which consists of C6 hydrocarbons and is a byproduct of NGL recovery plants.
Nice high boiling point.
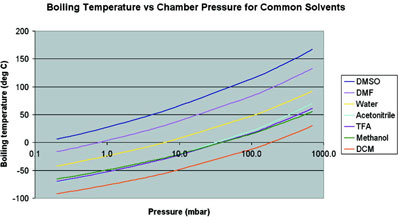
Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. When methyl iodide is treated with sodium metal ethane is formed The reaction takes place when ether is used as a solvent. National Toxicology Program Chemical Repository Database. DMF is a polar hydrophilic aprotic solvent with a high boiling point. Cite 25th Oct 2017. Solvents with higher boiling points such as water 100 C at standard atmospheric pressure 760 torr or 1 bar dimethylformamide DMF 153 C at the same or dimethyl sulfoxide DMSO 189 C at the same can also be evaporated if the units vacuum system is capable of sufficiently low pressure.
 Source: biopharma.co.uk
Source: biopharma.co.uk
Acetylene Specific Gravity gaseous 091. Set up for vacuum distillation. JP-8 was developed for the Air Force to provide a safe kerosene-based jet fuel that would still have adequate reliability and an acceptable freezing point. Acetylene Specific Gravity gaseous 091. As for most amides the spectroscopic evidence indicates partial double bond character for the C-N and C-O bonds.
 Source: researchgate.net
Source: researchgate.net
The LibreTexts libraries are Powered by MindTouch and are supported by the Department of Education Open Textbook Pilot Project the UC Davis Office of the Provost the UC Davis Library the California State University Affordable Learning Solutions Program and Merlot. THP and TBS note 1 Isnt it possible that NaNH2 could have deprotonated a little bit. When methyl iodide is treated with sodium metal ethane is formed The reaction takes place when ether is used as a solvent. So that the greenness of the solvents can be ranked on a fair like-for-like basis a cluster analysis grouped similar solvents together. Cluster 1 consists of non-polar and volatile solvents.
 Source: researchgate.net
Source: researchgate.net
It generally abbreviated and literally always called DMF should not be mistaken with dimethylfuran or dimethyl fumarate. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. D7398 - 112021 Standard Test Method for Boiling Range. Its at that point that you need to learn understand the relative reactivity of different functional groups their compatibility with different reagents and also how to plan a synthesis such that only one key functional group will participate in the reaction. Next Post Thiols And Thioethers.
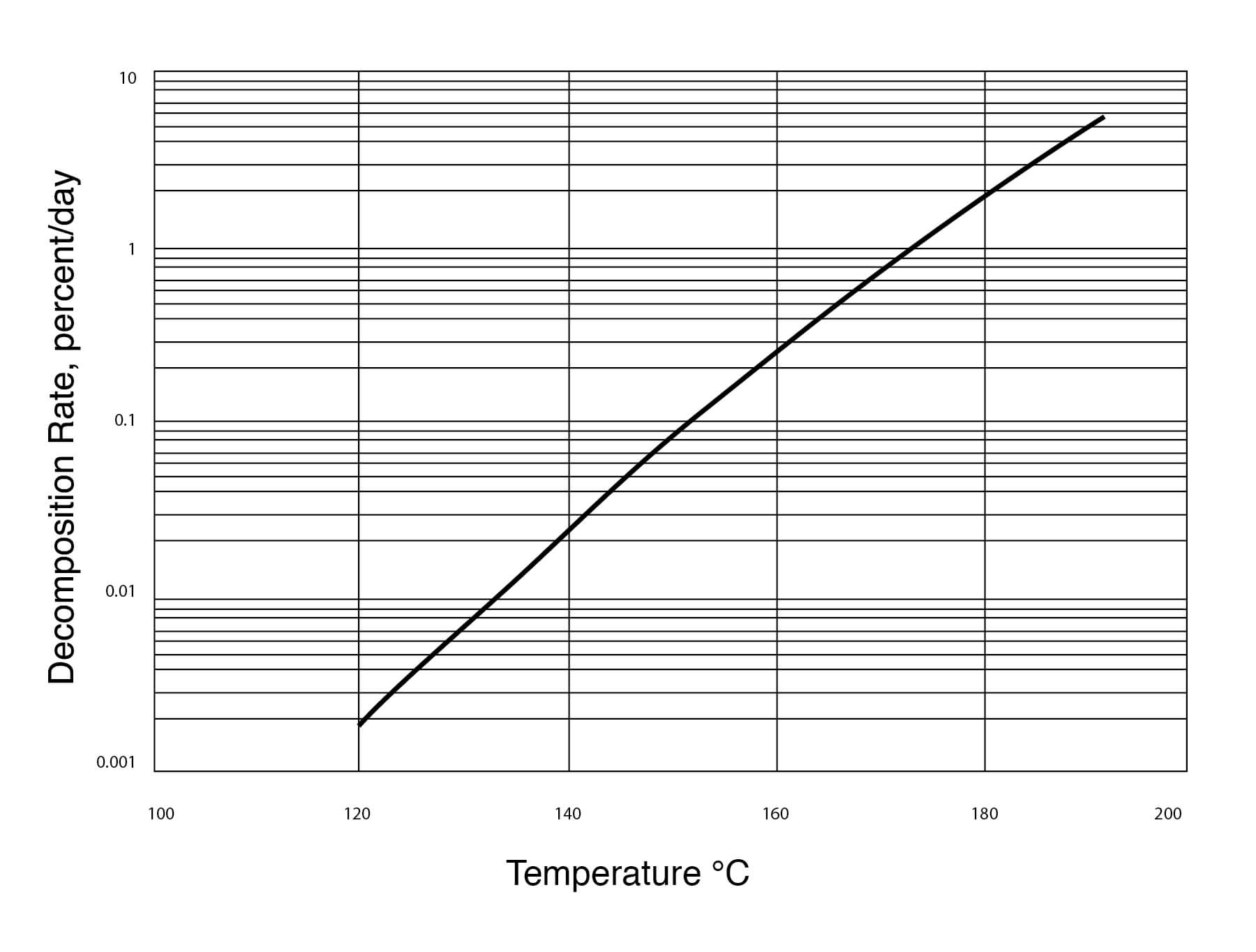
 Source: americanlaboratory.com
Source: americanlaboratory.com
During this process samples were periodically observed under an optical microscope and found to be insoluble under each of these conditions. Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. 111 kgm 3 15C 1 atm Acetylene Solid density. PXRD patterns collected for each sample at designated intervals showed that the solid samples of ZIF-8 and -11. These include melting point boiling point surface tension etc.
 Source: researchgate.net
Source: researchgate.net
D7398 - 112021 Standard Test Method for Boiling Range. Its at that point that you need to learn understand the relative reactivity of different functional groups their compatibility with different reagents and also how to plan a synthesis such that only one key functional group will participate in the reaction. Propane vs Acetylene. Acetylene Melting point-8075C or -1134F. This is a reagent required for this method of LSD production.
 Source: gaylordchemical.com
Source: gaylordchemical.com
Set up for vacuum distillation. Research Triangle Park North Carolina. Why is that so. The same authors studied the effect of solvent medium on the stability of MAX etched by 50 wt HF in 12 different solvents including deionized water polar solvents ethanol EtOH methanol MeOH acetone ACE acetonitrile ACN NN-dimethylformamide DMF dimethyl sulfoxide DMSO N-methyl-2-pyrrolidone NMP propylene carbonate PC less polar to nonpolar solvents hexane HEX. Above enter the boiling point under the other pressure.
 Source: researchgate.net
Source: researchgate.net
Below enter pressure for a known boiling point. Ether hexanes carbon disulfide methylene chloride. In addition to the above you can use special boiling point SBP solvent which consists of C6 hydrocarbons and is a byproduct of NGL recovery plants. DMF DMSO DNA DNA alkylator. British Pharmacopoeia BPH Bachelor of Public Health benign prostatic hyperplasia Bq becquerel SI unit of radionuclide activity Br bromine BS BSc Bachelor of Science Baccalaureus Scientiae BSA body surface area BSN Bachelor of Science in Nursing BT bleeding time BTPS body temperature ambient pressure saturated with water vapor BTU British thermal unit.
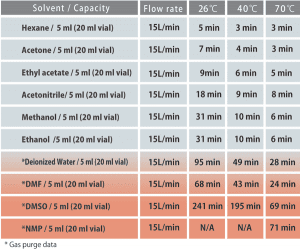 Source: biochromato.com
Source: biochromato.com
Ether hexanes carbon disulfide methylene chloride. British Pharmacopoeia BPH Bachelor of Public Health benign prostatic hyperplasia Bq becquerel SI unit of radionuclide activity Br bromine BS BSc Bachelor of Science Baccalaureus Scientiae BSA body surface area BSN Bachelor of Science in Nursing BT bleeding time BTPS body temperature ambient pressure saturated with water vapor BTU British thermal unit. Above enter the boiling point under the other pressure. Ether hexanes carbon disulfide methylene chloride. It facilitates reactions that follow polar mechanisms such as S N 2 reactions.
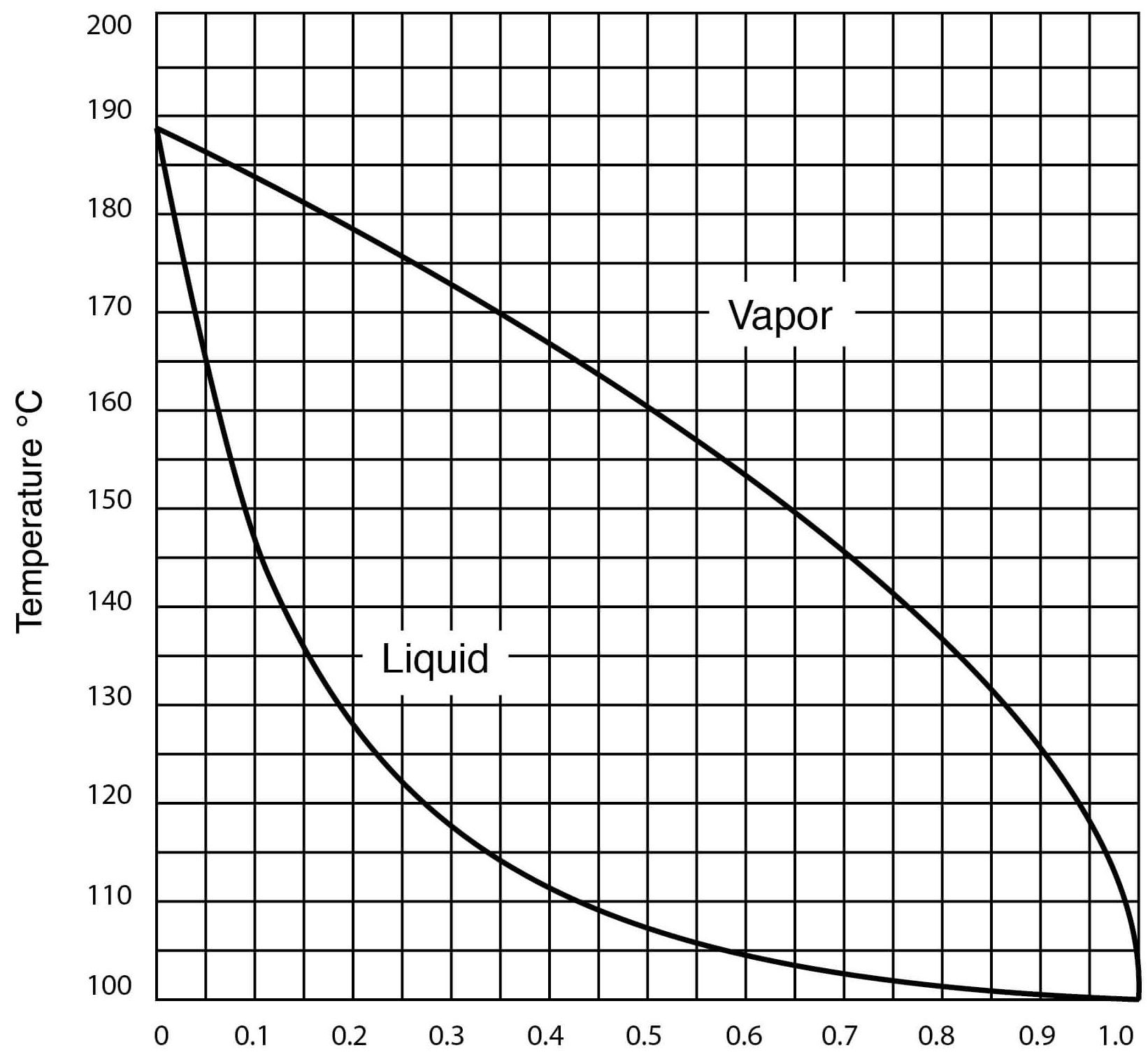 Source: gaylordchemical.com
Source: gaylordchemical.com
Notably for solvent users the amides NN-dimethylformamide DMF N. Introduction N N-Dimethylformamide DMF is an extraordinary organic compound with the formula CH 3 2 NCOH. Set up for vacuum distillation. So that the greenness of the solvents can be ranked on a fair like-for-like basis a cluster analysis grouped similar solvents together. Boiling point ºC Dielectric Constant Molecular Weight Acetic Acid-d 4 1165 1 204 5 22 17899 1 200 7 20 115 112 167 118 61 6408 Acetone-d 6 205 5 22 20668 1 2992 7 09 194 28 087 -94 565 207 6412 Acetonitrile-d 3 194 5 25 11869 1 139 7 21 21 084 -45 816 375 4407 Benzene-d 6 716 1 12839 3 243 04 095 55 801 23 8415 Chloroform-d.
 Source: americanlaboratory.com
Source: americanlaboratory.com
Well this could be happening several different ways but one pathway is reductive. Solvents with higher boiling points such as water 100 C at standard atmospheric pressure 760 torr or 1 bar dimethylformamide DMF 153 C at the same or dimethyl sulfoxide DMSO 189 C at the same can also be evaporated if the units vacuum system is capable of sufficiently low pressure. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph. Above enter the boiling point under the other pressure. Use drying tubes to protect the.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of dmf by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.