Boiling point of dioxane
Home » datasheet » Boiling point of dioxaneBoiling point of dioxane
Boiling Point Of Dioxane. You can use a double boiler hot plate or slow cooker to achieve this. Azeotropes consist of two three or more components and can be homogeneous or heterogeneous more than one phase 54Abbott researchers have detailed using the water contents and solvent concentrations using azeotropes in chasing H 2 O and i-PrOAc with i-PrOH 55. 14-Dioxane 1033 Acetic acid 1049 Acetic anhydride 108 Dimethyl sulfoxide 1092 Chlorobenzene 11066 Deuterium oxide 1107 Ethylene glycol 1115 Diethylene glycol 1118 Propylene carbonate 121 Formic acid 122 12-Dichloroethane 1245 Glycerin 1. Forensic investigators use luminol to detect trace amounts of blood at crime scenes as it reacts with the iron in hemoglobin.
 1 4 Dioxane Wikipedia From en.wikipedia.org
1 4 Dioxane Wikipedia From en.wikipedia.org
Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agentLuminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents but insoluble in water. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. 14-Dioxane 1033 Acetic acid 1049 Acetic anhydride 108 Dimethyl sulfoxide 1092 Chlorobenzene 11066 Deuterium oxide 1107 Ethylene glycol 1115 Diethylene glycol 1118 Propylene carbonate 121 Formic acid 122 12-Dichloroethane 1245 Glycerin 1. Forensic investigators use luminol to detect trace amounts of blood at crime scenes as it reacts with the iron in hemoglobin. Keep the heat setting on low it shouldnt be boiling. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the.
Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agentLuminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents but insoluble in water.
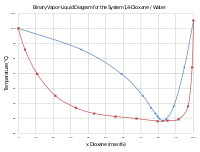
Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. Keep the heat setting on low it shouldnt be boiling. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. 14-Dioxane 1033 Acetic acid 1049 Acetic anhydride 108 Dimethyl sulfoxide 1092 Chlorobenzene 11066 Deuterium oxide 1107 Ethylene glycol 1115 Diethylene glycol 1118 Propylene carbonate 121 Formic acid 122 12-Dichloroethane 1245 Glycerin 1. Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agentLuminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents but insoluble in water. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components.
 Source: wikiwand.com
Source: wikiwand.com
The boiling point is an important property because it determines the speed of evaporation. Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agentLuminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents but insoluble in water. Azeotropes consist of two three or more components and can be homogeneous or heterogeneous more than one phase 54Abbott researchers have detailed using the water contents and solvent concentrations using azeotropes in chasing H 2 O and i-PrOAc with i-PrOH 55. You can use a double boiler hot plate or slow cooker to achieve this. 14-Dioxane 1033 Acetic acid 1049 Acetic anhydride 108 Dimethyl sulfoxide 1092 Chlorobenzene 11066 Deuterium oxide 1107 Ethylene glycol 1115 Diethylene glycol 1118 Propylene carbonate 121 Formic acid 122 12-Dichloroethane 1245 Glycerin 1.

Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. Keep the heat setting on low it shouldnt be boiling. After a half-hour or so you can strain the plant. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. After a half-hour or so you can strain the plant. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Azeotropes consist of two three or more components and can be homogeneous or heterogeneous more than one phase 54Abbott researchers have detailed using the water contents and solvent concentrations using azeotropes in chasing H 2 O and i-PrOAc with i-PrOH 55. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. Azeotropes consist of two three or more components and can be homogeneous or heterogeneous more than one phase 54Abbott researchers have detailed using the water contents and solvent concentrations using azeotropes in chasing H 2 O and i-PrOAc with i-PrOH 55. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components. After a half-hour or so you can strain the plant.

Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agentLuminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents but insoluble in water. The boiling point is an important property because it determines the speed of evaporation. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Strain to Remove the Plant Material. 14-Dioxane 1033 Acetic acid 1049 Acetic anhydride 108 Dimethyl sulfoxide 1092 Chlorobenzene 11066 Deuterium oxide 1107 Ethylene glycol 1115 Diethylene glycol 1118 Propylene carbonate 121 Formic acid 122 12-Dichloroethane 1245 Glycerin 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Small amounts of low-boiling-point solvents like. Forensic investigators use luminol to detect trace amounts of blood at crime scenes as it reacts with the iron in hemoglobin. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder.

Forensic investigators use luminol to detect trace amounts of blood at crime scenes as it reacts with the iron in hemoglobin. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. 14-Dioxane 1033 Acetic acid 1049 Acetic anhydride 108 Dimethyl sulfoxide 1092 Chlorobenzene 11066 Deuterium oxide 1107 Ethylene glycol 1115 Diethylene glycol 1118 Propylene carbonate 121 Formic acid 122 12-Dichloroethane 1245 Glycerin 1. Small amounts of low-boiling-point solvents like. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components.
 Source: semanticscholar.org
Source: semanticscholar.org
After a half-hour or so you can strain the plant. Gently heat the solution for about 3 hours stirring frequently. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components. Small amounts of low-boiling-point solvents like.
You can use a double boiler hot plate or slow cooker to achieve this. You can use a double boiler hot plate or slow cooker to achieve this. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. Small amounts of low-boiling-point solvents like. An azeotrope is a constant-boiling mixture with a constant mole fraction composition of components.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Add near boiling distilled water 90ºC at a ratio of 1 mL water to 1 gram of dried root powder. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Keep the heat setting on low it shouldnt be boiling. Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agentLuminol is a white-to-pale-yellow crystalline solid that is soluble in most polar organic solvents but insoluble in water.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of dioxane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.