Boiling point of dimethyl ether
Home » datasheet » Boiling point of dimethyl etherBoiling point of dimethyl ether
Boiling Point Of Dimethyl Ether. Trimethylamine CH 3 3 N. Because of its high boiling point and affinity for water ethylene glycol is a useful desiccant. Its usefulness is limited by its low boiling point 23 C 9 F but the same property facilitates its removal from reaction mixtures. On account of its high evaporation number even additions of.
 Why Does Diethyl Ether Have A Higher Boiling Point Than Ethane Does Quora From quora.com
Why Does Diethyl Ether Have A Higher Boiling Point Than Ethane Does Quora From quora.com
Boiling Point - at saturation pressure 147 psia and. CH 3 CH 2 2 NH 2. Butyl diglycol is used as a high-boiling solvent to improve gloss and flow properties. Physical and thermal properties of acetone also called 2-propanone dimethyl ketone and pyroacetic acid. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. In cellulose nitrate and cellulose ether lacquers even smaller amounts are effective.
Boiling Point Melting Point.
Fuel Flash Point o F Acetaldehyde-36. These are isomers having the same molecular formula C 5 H 12 but differ in their structures. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. The growth of the dimethyl ether market is driven by burgeoning demand for eco-friendly transportation fuels soaring need to reduce carbon emissions and surging requirement of eco-friendly. Butyl diglycol is used as a high-boiling solvent to improve gloss and flow properties. Some liquids and their flash points at atmospheric pressure.
 Source: quora.com
Source: quora.com
CH 3 OCH 3. The flash point is an indication of how easy a chemical may burn. Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points. CH 3 CH 2 NHCH 3. We mentioned in the previous post that stronger intermolecular interactions increase the boiling and melting points but how exactly they affect the physical properties might be your next question.
 Source: differencebetween.com
Source: differencebetween.com
Because of its high boiling point and affinity for water ethylene glycol is a useful desiccant. Consider the boiling point of n-pentane and neo-pentane 22-dimethyl propane. CH 3 OCH 3. Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points. CH 3 CH 2 2 NH 2.
 Source: chegg.com
Source: chegg.com
Ethylene glycol is widely used to inhibit the formation of natural gas clathrates hydrates in long multiphase pipelines that convey natural gas from remote gas fields to a gas processing facility. Air - Density Specific Weight and Thermal Expansion Coefficient vs. CH 3 OCH 3. Boiling Point Melting Point. Trimethylamine CH 3 3 N.
 Source: youtube.com
Source: youtube.com
Boiling Point and Dipole-Dipole Interactions. Boiling Point - at saturation pressure 147 psia and. Boiling Point Melting Point. Its usefulness is limited by its low boiling point 23 C 9 F but the same property facilitates its removal from reaction mixtures. Butyl diglycol is used as a high-boiling solvent to improve gloss and flow properties.
 Source: youtube.com
Source: youtube.com
Butyl diglycol is used as a high-boiling solvent to improve gloss and flow properties. Some liquids and their flash points at atmospheric pressure. Fuel Flash Point o F Acetaldehyde-36. Consider the boiling point of n-pentane and neo-pentane 22-dimethyl propane. CH 3 CH 2 2 NH 2.
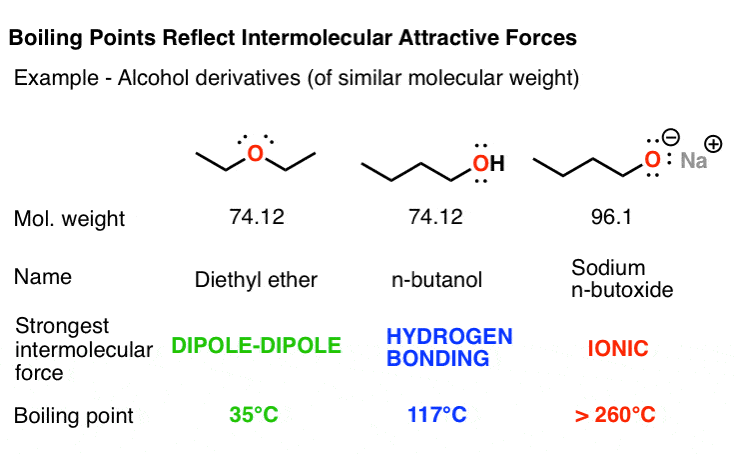 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
CH 3 CH 2 2 NH 2. Trimethylamine CH 3 3 N. Its usefulness is limited by its low boiling point 23 C 9 F but the same property facilitates its removal from reaction mixtures. CH 3 CH 2 2 OH. In cellulose nitrate and cellulose ether lacquers even smaller amounts are effective.
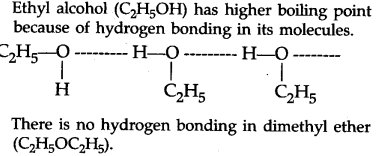 Source: ask.learncbse.in
Source: ask.learncbse.in
Some liquids and their flash points at atmospheric pressure. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points. Boiling Point Melting Point. CH 3 CH 2 OH.
Source: quora.com
These are isomers having the same molecular formula C 5 H 12 but differ in their structures. The boiling point of neopentane is much lower than that of n-pentane. Butyl diglycol is used as a high-boiling solvent to improve gloss and flow properties. In emulsion paints and. Dimethyl ether is a low-temperature solvent and extraction agent applicable to specialised laboratory procedures.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
In emulsion paints and. Diethyl ether CH 3 CH 2 2 O. See also Autoignition temperature and flash point of different hydrocarbons. The boiling point of neopentane is much lower than that of n-pentane. Some liquids and their flash points at atmospheric pressure.
 Source: dietosa.blogspot.com
Source: dietosa.blogspot.com
Dimethyl ether is a low-temperature solvent and extraction agent applicable to specialised laboratory procedures. Air - Density Specific Weight and Thermal Expansion Coefficient vs. Its usefulness is limited by its low boiling point 23 C 9 F but the same property facilitates its removal from reaction mixtures. CH 3 CH 2 OH. The boiling point of neopentane is much lower than that of n-pentane.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of dimethyl ether by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.