Boiling point of diethyl ether
Home » datasheet » Boiling point of diethyl etherBoiling point of diethyl ether
Boiling Point Of Diethyl Ether. By weight in mixture. 667 g100 ml Solubility. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. After completion of the reaction sodium chloride and catalyst are separated.
 Diethyl Ether Has A Normal Boiling Point Of 35 0 C And Has An Entropy Of Vaporization Of 84 4 Youtube From youtube.com
Diethyl Ether Has A Normal Boiling Point Of 35 0 C And Has An Entropy Of Vaporization Of 84 4 Youtube From youtube.com
Fuel Flash Point o F Acetaldehyde-36. The solvent evaporates under the vacuum and the dissolved solids precipitate clogging up the pores of the filter paper and get stuck on the insides of the funnel ie. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. Diethyl ethyl ether. Ether is a volatile flammable colourless liquid with a distinctive odour. Materials with higher flash points are less flammable or hazardous than chemicals with lower flash points.
The chemical formula for diethyl ether is C 2 H 5 OC 2 H 5.
473 K Solubility in water. Posted February 6 2014. Ether is a volatile flammable colourless liquid with a distinctive odour. The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. Three multi-investigator groups that operate principally in the TBHIV space. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample.
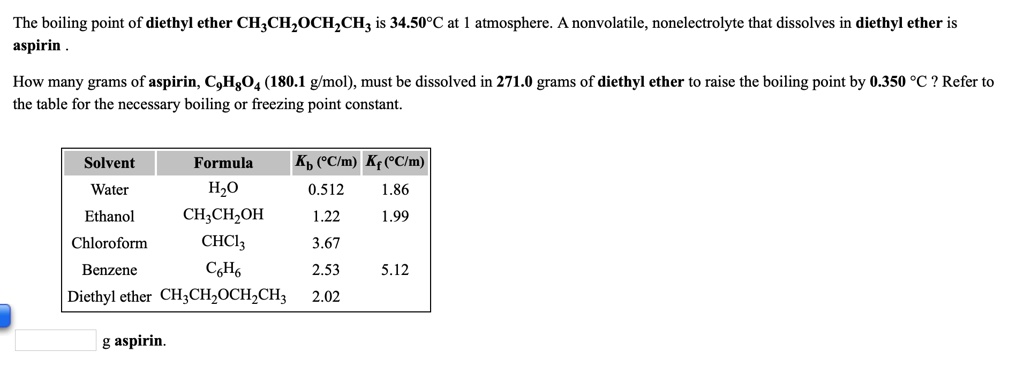 Source: numerade.com
Source: numerade.com
ZCOSE DOCSIIPPWritten ProgramsChemical Hygien e PlanFlammable and Combustible Liquids_CHPdoc Page 1 of 2. Alcohol - ethyl grain ethanol C. The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. Gardless of amount. Glycol ethers are a group of solvents based on alkyl ethers of ethylene glycol or propylene glycol commonly used in paints and cleaners.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Ether is a common name for diethyl ether. Fuel Flash Point o F Acetaldehyde-36. It belongs to the large group of organic compounds called ethers. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. 473 K Solubility in water.
 Source: quora.com
Source: quora.com
It is the most common ether known. Triclinic Hazards Safety data sheet. Posted February 6 2014. Glycol ethers are a group of solvents based on alkyl ethers of ethylene glycol or propylene glycol commonly used in paints and cleaners. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below.
 Source: youtube.com
Source: youtube.com
What is Diethyl Ether. Some liquids and their flash points at atmospheric pressure. Must ALWAYS be stored in approved flammable storage cabinet re. Acidity pK a 6 Magnetic susceptibility χ-79910 6 cm 3 mol Structure Crystal structure. Triclinic Hazards Safety data sheet.
 Source: youtube.com
Source: youtube.com
That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. Congratulations to my chairman Dr Vaughn Starnes 100th AATS. The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample.
Source: chemspider.com
After completion of the reaction sodium chloride and catalyst are separated. Three multi-investigator groups that operate principally in the TBHIV space. It belongs to the large group of organic compounds called ethers. The vapor pressure of a liquid varies with its temperature as the following graph shows for water. Triclinic Hazards Safety data sheet.
 Source: clutchprep.com
Source: clutchprep.com
Alcohol - ethyl grain ethanol C. Gardless of amount. Diethyl ethyl ether. See also Autoignition temperature and flash point of different hydrocarbons. Acetone CH 3 COCH 3.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
A Misguided Idea The truth behind the universal but flawed catchphrase for creativity. The Wellcome Centre for Infectious Diseases Research in Africa CIDRI-Africa which includes Robert Wilkinson Director Graeme Meintjes Catherine Riou and Anna Coussens. See also Autoignition temperature and flash point of different hydrocarbons. By weight in mixture. Class IB Flash point below 73 F boiling point at or above 100 F.
Source: quora.com
The vapor pressure of a liquid varies with its temperature as the following graph shows for water. It belongs to the large group of organic compounds called ethers. As the temperature of a liquid or solid increases its vapor pressure also increases. The South African TB Vaccine Initiative SATVI which includes Mark Hatherill Director Tom Scriba Deputy Director and Elisa Nemes. Thinking Outside the Box.
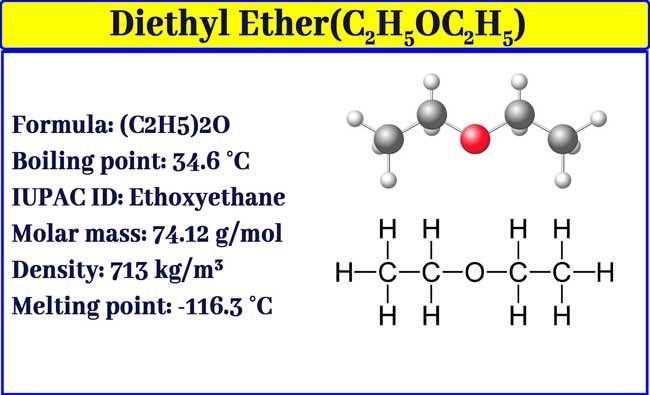 Source: chemistrypage.in
Source: chemistrypage.in
In the case of diethyl malonate the reaction is conducted at around 100 C and 18 bar at pH 57. Gardless of amount. The South African TB Vaccine Initiative SATVI which includes Mark Hatherill Director Tom Scriba Deputy Director and Elisa Nemes. Diethyl ethyl ether. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of diethyl ether by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.